Residual DNA detection assays
Our bioprocess quality control solutions are designed to streamline your host cell residual DNA detection workflow, from sample extraction to qPCR quantification. Helping you achieve the purity of your biotherapeutic and CGT products with assays that can demonstrate whether contaminant levels are in compliance with critical quality attributes.

HostDetect CHO PCR DNA Quant Kit
The HostDetect CHO PCR DNA Quant Kit utilizes sequence-specific primers and TaqMan® probe to amplify the Alu-equivalent gene of CHO genomic DNA for residual host genomic identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products.
This kit is specific for DNA from the CHO genome and cannot detect human or environmental DNA that might be introduced during sample handling.
Key Highlights:
- Quantify CHO genomic DNA with as little as 0.01 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit

HostDetect HEK293 PCR DNA Quant Kit
The HostDetect HEK293 PCR DNA Quant Kit utilizes sequence-specific primers and TaqMan® probe to amplify the Alu-equivalent gene of HEK293 genomic DNA for residual host DNA identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products.
This kit is specific for DNA from the HEK293 genome and cannot detect human or environmental DNA that might be introduced during sample handling.
For more information, contact: BioprocessQC@revvity.com
Key highlights:
- Quantify HEK293 genomic DNA with as little as 0.03 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit

HostDetect E.coli PCR DNA Quant Kit
The HostDetect E. coli PCR DNA Quant Kit utilizes sequence-specific primers and TaqMan® probe to amplify the 16S ribosomal RNA gene of the E. coli genome for residual host DNA identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products.
This kit is specific for DNA from the E. coli genome and cannot detect human or environmental DNA that might be introduced during sample handling.
For more information, contact: BioprocessQC@revvity.com
Key highlights:
- Quantify E.coli genomic DNA with as little as 0.03 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit
Host cell protein detection assays
During biotherapeutic manufacturing and production, CHO, HEK 293 and E.coli host cells produce HCP impurities which, if not removed, can induce immunogenicity in individuals or reduce the potency, stability, or effectiveness of a drug. Therefore, to meet regulatory organizations’ guidelines we have developed a range of HCP detection assays to improve your workflows.

HTRF CHO HCP Detection Kit, 500 Assay Points
The CHO Host Cell Protein (HCP) kit measures contaminants originating from the CHO cells used to manufacture therapeutic antibodies. The kit can be used for the quantification of CHO HCP proteins in routine bioprocess operations, from the completely raw harvest material to the final product. The simple and robust procedure benefits from increased throughput compared to ELISA.
HTRF assays offer many advantages over other technologies:
- Homogeneous add-and-read format
- No wash steps
- Low background
- Straightforward miniaturization from 96- or 384-well microplates to high density assay formats such as 384-well low volume and 1536-well plates
- Stable signal, providing flexibility in time of readout or size of assays
AlphaLISA HEK 293 HCP Detection Kit, 100 Assay Points
Human Embryonic Kidney (HEK) 293 is a cell line commonly used for research and biopharmaceutical production. The HEK Host Cell Proteins (HCP) kit is designed to measure contaminants originating from the HEK cells used to manufacture therapeutic antibodies. This kit can be utilized to quantify HEK HCP proteins at various stages, from routine bioprocess operations to crude harvest materials to final products. Our simple procedure and robust assay offer increased throughput compared to ELISA.
Formats
- Our 100 assay point kit allows you to run 100 wells in 96-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 500 assay point kit allows you to run 500 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 5,000 assay point kit allows you to run 5,000 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
Features
- No-wash steps, no separation steps
- ELISA alternative technology
- Sensitive detection
- Broad sample compatibility
- Small sample volume
- Results in less than 4 hours
- Half the time of an ELISA assay
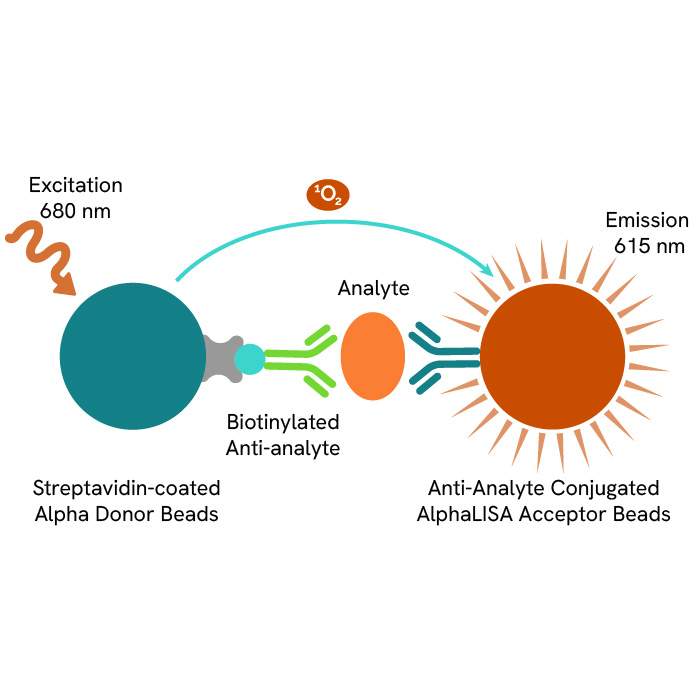
AlphaLISA technology allows the detection of molecules of interest in a no-wash, highly sensitive, quantitative assay. In an AlphaLISA assay, a biotinylated anti-analyte antibody binds to the Streptavidin-coated Donor beads while another anti-analyte antibody is conjugated to AlphaLISA Acceptor beads. In the presence of the analyte, the beads come into close proximity. The excitation of the Donor beads causes the release of singlet oxygen molecules that triggers a cascade of energy transfer in the Acceptor beads, resulting in a sharp peak of light emission at 615 nm.

AlphaLISA CHO HCP Detection Kit, 100 Assay Points
CHO cells are widely used expression hosts for recombinant proteins and are utilized for the generation of monoclonal antibodies. The CHO Host Cell Proteins (HCP) kit is designed to measure contaminants originating from the CHO cells used to manufacture therapeutic antibodies. This kit can be utilized to quantify CHO HCP proteins at various stages, from routine bioprocess operations to crude harvest materials to final products. Our simple procedure and robust assay offer increased throughput compared to ELISA.
Formats:
- Our 100 assay point kit allows you to run 100 wells in 96-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 500 assay point kit allows you to run 500 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 5,000 assay point kit allows you to run 5,000 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
Features:
- No-wash steps, no separation steps
- ELISA alternative technology
- Sensitive detection
- Broad sample compatibility
- Small sample volume
- Results in less than 3 hours
- Half the time of an ELISA assay
AlphaLISA technology allows the detection of molecules of interest in a no-wash, highly sensitive, quantitative assay. In an AlphaLISA assay, a biotinylated anti-analyte antibody binds to the Streptavidin-coated Donor beads while another anti-analyte antibody is conjugated to AlphaLISA Acceptor beads. In the presence of the analyte, the beads come into close proximity. The excitation of the Donor beads causes the release of singlet oxygen molecules that triggers a cascade of energy transfer in the Acceptor beads, resulting in a sharp peak of light emission at 615 nm.
Protein contamination detection technology
To aid rapid protein contamination detection, utilize an automated microfluidic analysis platform that can support FDA 21 CFR Part 11 regulations. By providing quantitative results for contaminants while using minimal sample volumes, the LabChip™ protein characterization system is invaluable in the biopharmaceutical manufacturing workflow by significantly enhancing protein purity assessment and helping you realize higher quality standards.

LabChip GXII Touch HTタンパク質特性評価システム
LabChip GXII Touchは、サンプル解析を簡素化します
ユーザーフレンドリーな操作:
- サンプルプレートとチップをロード
- サンプルを選択(1回のランで最大384サンプル)
- アッセイタイプを選択
- 『Run』をタッチして開始
- データは自動的にネットワークやLIMSにエクスポート可能
リアルタイムでランを確認:
- 最短42秒でサンプル析
- サンプルの泳動中にエレクトロフェログラムをリアルタイムで表示
- 取得データを重ねてサンプルプロファイルを比較
- 様々なランタイム解析機能の注釈を選択
データをリアルタイムで確認または後の解析のためにエクスポート:
- 表示形式はエレクトロフェログラム、バーチャルゲル、データテーブルから選択(Figure 1)
- 保存済みプレートを複数呼び出してレビューや比較解析
- 主要属性にフィルターをかけてデータマイニング
- 予想されるピークをハイライト
- FDA 21 CFR Part 11に対応したソフトウェアで関連するユーザーアクセスおよびデータ履歴パラメータを管理
タンパク質および核酸の特性評価のための簡単なセットアップ
LabChip GXII Touchタンパク質特性評価システムの操作コントロールは、ユーザーがわずか3つの簡単なステップで設定とランを行えるよう設計されています。テンプレートは、オペレーター制御機能とともにインポートすることもできます。ランテンプレートには、ウェルの選択、サンプル名、ピークテーブルなどを含めることができ、操作性が向上します。データはネットワークやLIMSディレクトリに自動エクスポートされ、その後の解析に利用できます。すべての装置には、現在のデータや保存済みデータの解析が可能なレビュー用ソフトウェアが標準で付属しています。
品質設計(Quality by Design)をワークフローに統合しやすい設計
LabChip GXII Touchタンパク質特性評価システムは、バイオ医薬品およびゲノム解析のワークフロー全体において、迅速な定量と品質管理を可能にします。たとえば、特性評価プロセスを自動化することで、多くの重要な品質属性を迅速に取得することが可能になります。研究者は、プロセスの初期段階で最適なタンパク質特性をスクリーニングし、品質設計(Quality by Design)イニシアチブをバイオ治療薬の開発ワークフローに統合することが可能です。
LabChip GXII Touchタンパク質特性評価システムにはFDA 21 CFR Part 11に対応したソフトウェアが利用可能
LabChip GXII TouchおよびReviewerソフトウェアにはFDA 21 CFR Part 11規制をサポートするために特別に設計された技術的コントロールおよび機能が内蔵されています。これらの機能には、共有ユーザーアカウントデータベース、アクセス制御、デバイスチェック、ランステップの強制的な順序付け、監査証跡、記録のコピー、記録の保存、システム文書化、および電子署名の制御が含まれます。
お客様のコンプライアンス計画を支援するために、LabChip GxP Security Softwareが利用可能です。このソフトウェアを使用することで、LabChip GXII Touchタンパク質特性解析システムをCFR21 Part 11準拠および検証に対応した環境で運用することができます(図2)。これには、アクセスセキュリティ、データセキュリティと検証、そして完全な監査ログ機能が含まれています。
LabChip CE: その仕組みは?
LabChip CEは小型のマイクロフルイディックチップ上で行われます。解析前に、チップの個別のウェルに試薬をロードします。これらのウェルは、クオーツ製のマイクロフルイディックチップに刻まれた人間の髪の毛の太さ程度の微小チャネルに接続されています。チップをLabChip GXII Touchタンパク質特性評価システムにロードすると、チップのウェルが白金電極と接触し、電圧と電流制御が提供されます。装置はマイクロタイタープレートを直接チップのキャピラリー『シッパー』の下に移動し、約150 nLのサンプルがチップに吸引されます。サンプルの染色および脱染はプラットフォーム上で自動的に実行されます。個々のサンプル解析物は電気泳動的に分離され、チップのキャピラリー内でレーザー誘導蛍光によってバンドが検出されます。各バンドのサイズおよび濃度は、ラダーおよび内部マーカーを使用して決定されます。サンプル間でシッパーをすすぐことでクロスコンタミネーションを最小化します。