
pHSense Eu SNAP Labeling Reagent, 96 wells
| Feature | Specification |
|---|---|
| Application | 内部化 |
| Sample Volume | 50 µL |



Product information
Overview
The pHSense™ Eu SNAP Labeling Reagent is non cell permeant, and is labeled with a pH sensitive europium complex that specifically and covalently interacts with SNAP-tag® receptors and membrane proteins to monitor their internalization. Cells expressing receptors and membrane proteins tagged with a SNAP-tag on the extracellular part can be labeled with pHSense™ Eu SNAP Labeling Reagent and remain minimally fluorescent at neutral extracellular pH (≥7). Once internalized, the pHSense™ Eu SNAP Labeling Reagent encounters increasingly acidic compartments such as early and late endosomes and lysosomes, where the europium signal becomes progressively stronger. This new pH sensitive europium SNAP-labeling reagent is compatible with a time-resolved fluorescence (TRF) detection, effectively eliminating most fluorescence background and significantly enhancing the signal-to-background ratio. Its unique photophysical properties enable simple and robust no-wash detection of receptor-mediated endocytosis in plate-based assays with live-cells.
How it works
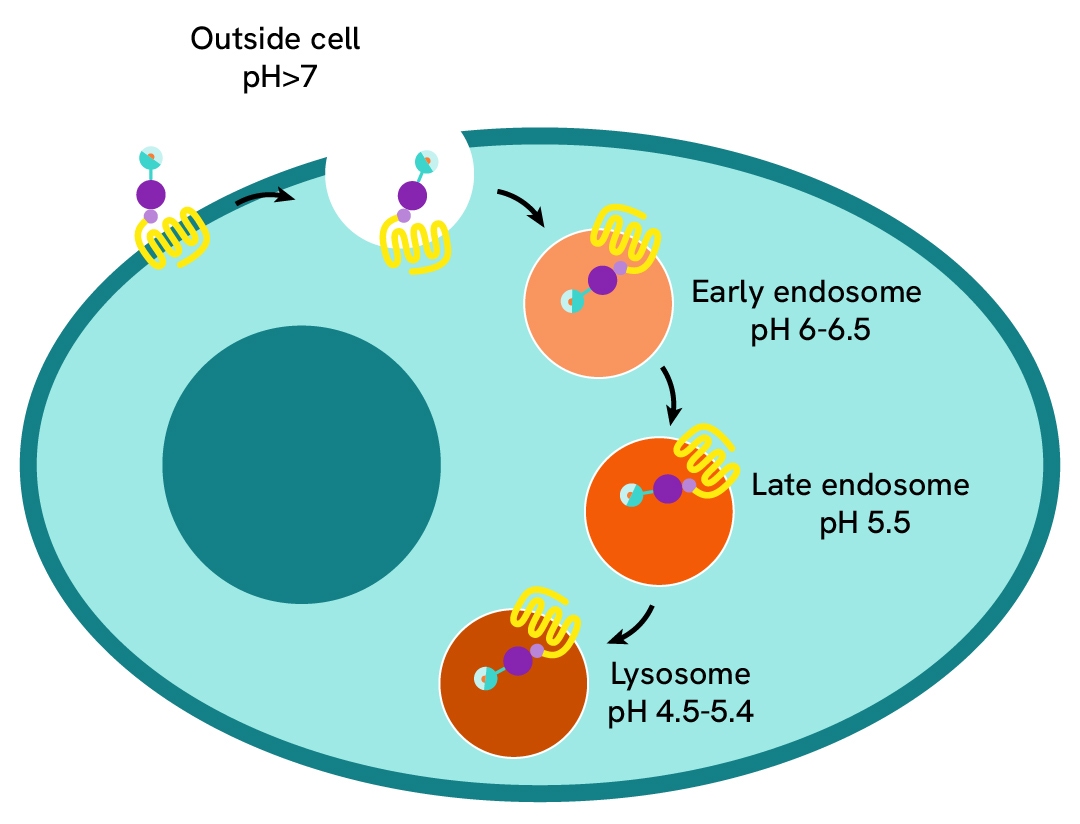
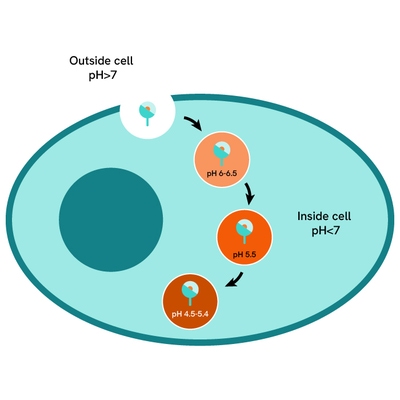
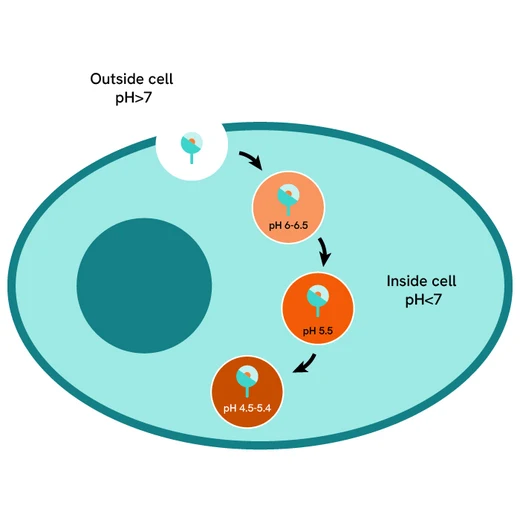
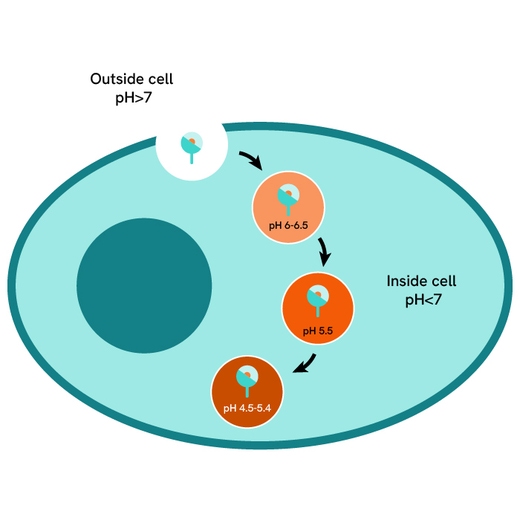
pHSense Eu SNAP labeling reagent assay principle
pHSense Eu SNAP Labeling Reagent is non cell permeant, and is labeled with a pH sensitive europium complex that specifically and covalently interacts with SNAP-tag® receptors and membrane proteins designed to monitor their internalization. Cells expressing receptors and membrane proteins tagged with a SNAP-tag on the extracellular part can be labeled with pHSense Eu SNAP Labeling Reagent, and remain minimally fluorescent at neutral extracellular pH (≥7). Once internalized, the pHSense Eu SNAP Labeling Reagent encounters increasingly acidic compartments such as early and late endosomes and lysosomes, where the europium fluorescent signal becomes progressively stronger.

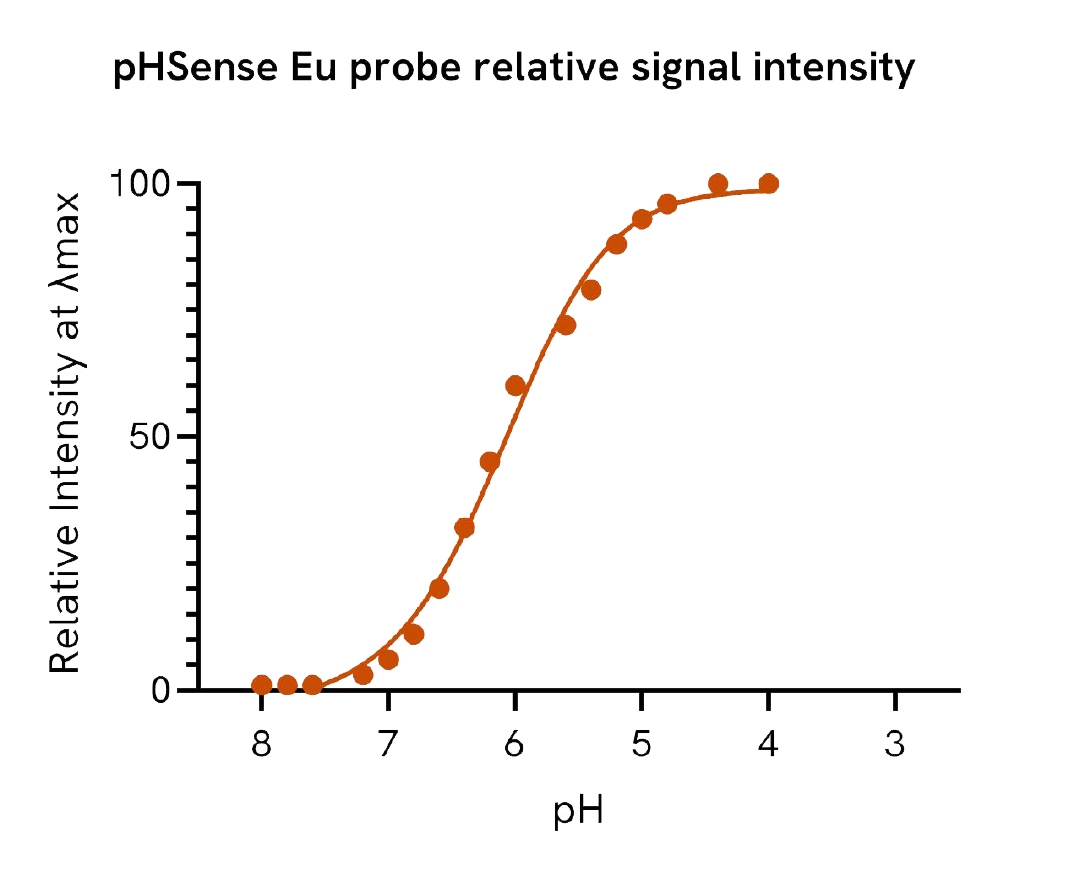
pHSense Eu SNAP labeling reagent assay protocol
The assay begins by culturing cells in a 96-well plate. The pHSense SNAP labeling reagent is then added to the cells and incubated for 1 hour at room temperature. Following this incubation, cells are stimulated with a pharmacological compound. Fluorescence is then measured either kinetically or at endpoint using an HTRF-compatible plate reader.
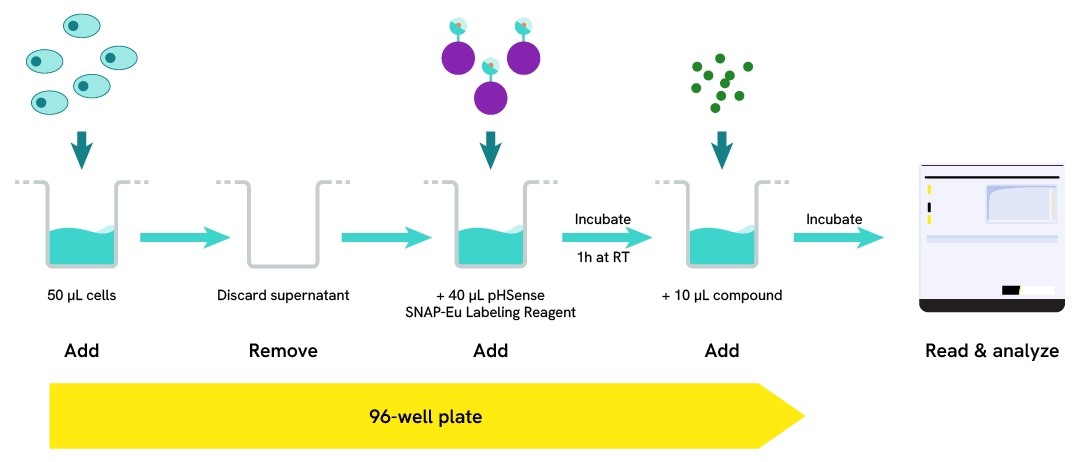
Assay validation
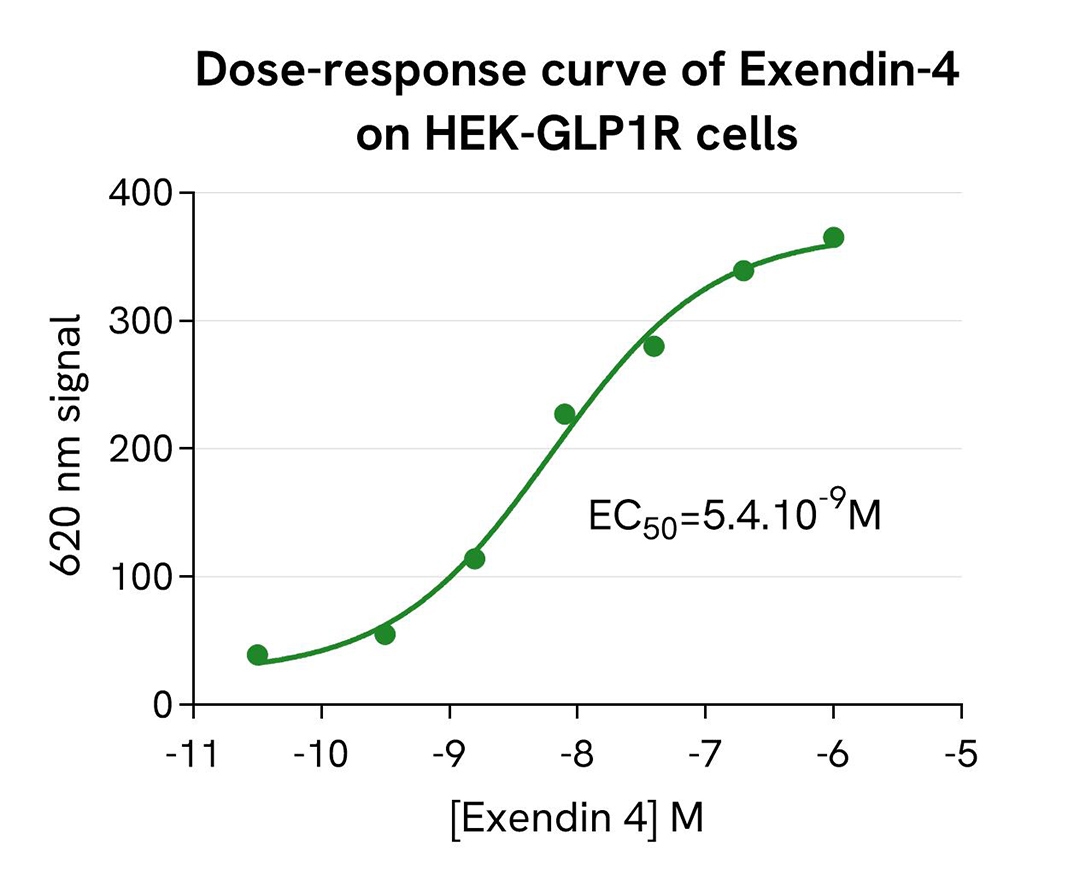
pHSense-based detection of GLP1R internalization following Exendin-4 stimulation
Tag-Lite® GLP1R cells (stable cell line, Part #: C1SU1GLP1, Revvity) were seeded in a 96-well white culture-treated plate at a density of 80,000 cells per well in complete culture medium, and then incubated overnight at 37°C with 5% CO₂. After cell supernatant removal, 40 µL of the pHSense Eu SNAP Labeling Reagent diluted in cell culture medium (DMEM +10%FBS) were added to the cells, and then incubated for 1 hour at room temperature.
Exendin-4, a GLP1R agonist, was serially diluted in cell culture medium, and 10 µL of each dilution were added to the wells. After incubation at 37°C, the signal was recorded using an HTRF-compatible plate reader. Results show Exendin-4 induced a dose-dependent increase in signal, with an EC₅₀ value consistent with values described in the scientific literature.
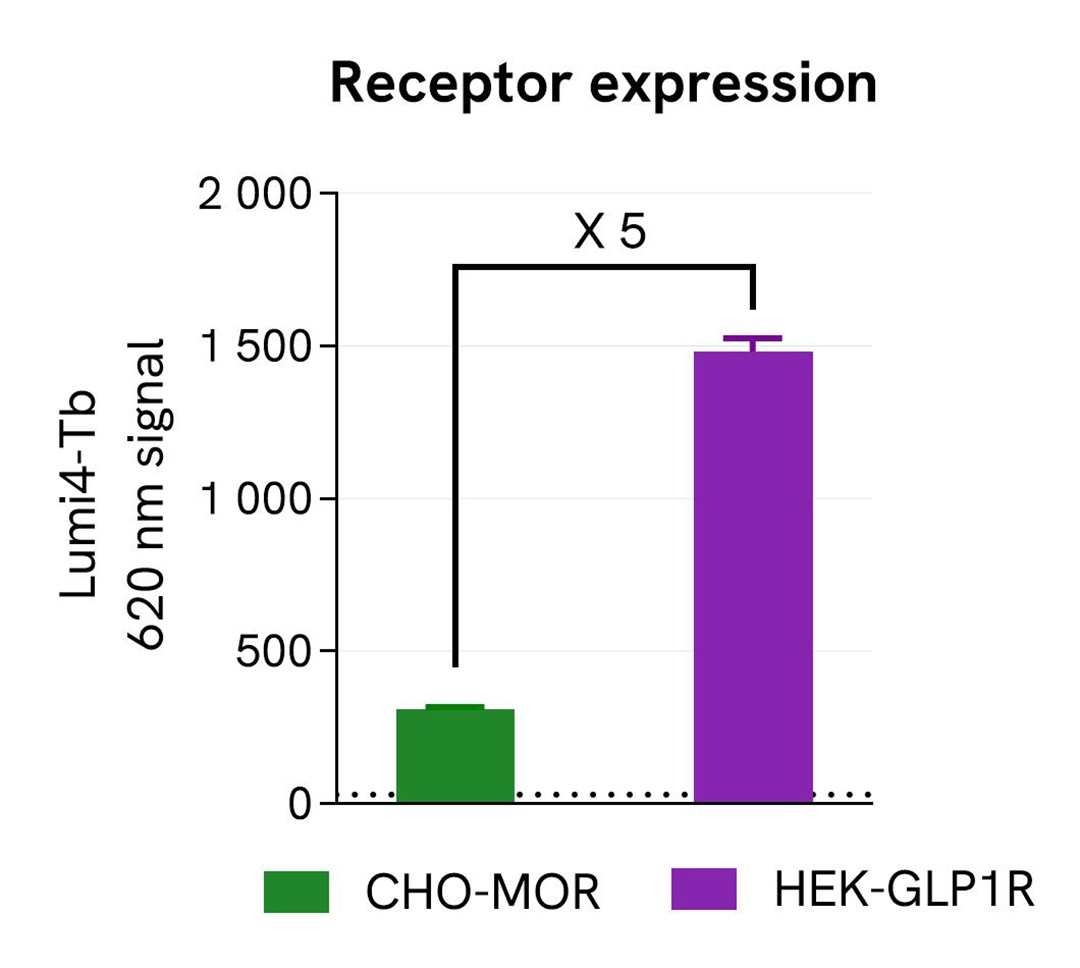
Validation of pHSense Eu SNAP labeling reagent assay in cell lines expressing different receptor levels
The pHSense Eu SNAP Labeling Reagent was tested on 2 different stable cell lines expressing various levels of receptors: Tag-Lite GLP1R cells (HEK-GLP1R) and Tag-lite Mu opioid cells (CHO-MOR).
The comparison of receptor expression levels was performed using the Tag-lite SNAP-Lumi4-Tb Labeling Reagent (Part#: SSNPTBC, Revvity). Cells were seeded in a 96-well black culture-treated plate at a density of 100,000 cells per well in complete culture medium. After overnight incubation at 37°C with 5% CO₂, the supernatant was removed and 100 µL of 100 nM SNAP-Lumi4-Tb Labeling Reagent were added to the cells, and then incubated 1h at 37°C with 5% CO₂. Cells were washed 3 times with Tag-lite SNAP/CLIP Labeling Medium 1X (Part#: LABMED, Revvity) before the addition of 100 µL of the same medium. The 620 nm signal was recorded using an HTRF-compatible plate reader. Results show Tag-Lite GLP1R cells exhibit 5-times higher expression levels than Tag-lite Mu opioid cells.
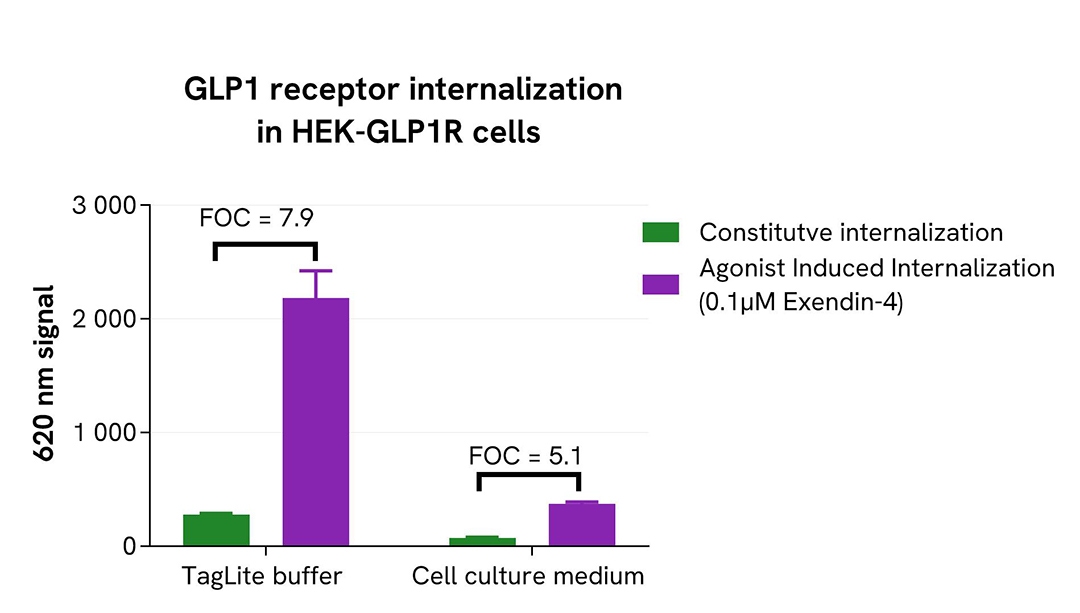
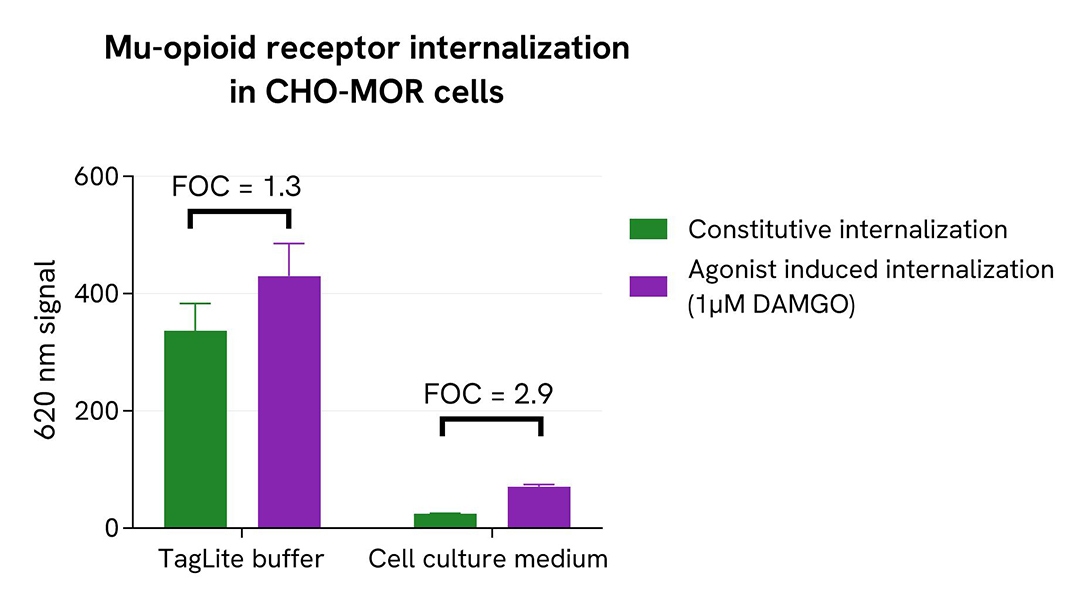
To perform pHSense Eu SNAP Labeling Reagent assay, Tag-Lite GLP1R and Tag-lite Mu opioid stable cell lines were seeded in a 96-well white culture-treated plate at a density of 80,000 cells per well in complete culture medium and incubated overnight at 37°C with 5% CO₂.
After cell supernatant removal, 40 µL of the pHSense Eu SNAP Labeling Reagent diluted in cell culture medium were added to the cells, and then incubated for 1 hour at room temperature. Exendin-4, a GLP1R agonist, and DAMGO, a Mu opioid agonist, were diluted in cell culture medium, and 10 µL of agonists or cell culture medium - as constitutive internalization condition - were added to the wells. After incubation at 37°C, the signal was recorded using an HTRF-compatible plate reader.
In parallel, the same assay was performed with pHSense Eu SNAP Labeling Reagent and receptor agonists prepared in Tag-lite SNAP/CLIP Labeling Medium as physiological buffer.
As demonstrated here, pHSense Eu SNAP Labeling Reagent allows the detection of agonist-induced and constitutive internalization in cell lines with different receptor expression levels, and is compatible with Tag-lite SNAP/CLIP Labeling Medium as physiological buffer or cell culture medium.
Specifications
| Application |
Internalization
|
|---|---|
| Brand |
pHSense
|
| Detection Modality |
pH sensitive dye
|
| Product Group |
Fluorescent Reagent
|
| Sample Volume |
50 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
SNAP-tag fusion protein
|
| Target Class |
Cell surface proteins, antibodies, ADCs
|
| Technology |
TRF
|
| Unit Size |
96 wells
|
Resources
Are you looking for resources, click on the resource type to explore further.
This techincal note explores how pHSense™ Eu TRF probes enable no-wash, live-cell detection of GPCR internalization across...


How can we help you?
We are here to answer your questions.