細胞療法の製品コストの削減
LentiBOOST™ 形質導入エンハンサーは、必要なベクター量を削減することで、細胞治療の製造コスト削減を実現します。マンチェスター大学およびUCLの研究者らは、GMP幹細胞製造プロトコルにおける造血幹細胞(HSCs)において、同等の効果を維持しながらベクター量を約5分の1に削減できることを実証しました(Ellison et al. 2024)。
治療効果の向上を支援
レンチウイルス導入効率をLentiBOOSTテクノロジーで向上させることは、治療効果の反応率を高める方法の一つです。例えば、、疲弊したT細胞を扱う自己移植の応用や、利用可能な生存T細胞が少ない場合、効果的なレンチウイルス導入は反応の有無を分ける要因となり得ます。また、大型の構築体を使用する場合にも同様のことが言えます。
レンチウイルス導入効率の向上と細胞あたりの最適コピー数
LentiBOOSTレンチウイルス形質導入エンハンサー試薬を使用することで、お客様は、初代T細胞や造血幹細胞のような導入困難とされている場合を含む、さまざまな細胞タイプで改善された遺伝子導入を実証しています。
この事例は、レンチウイルスとLentiBOOSTテクノロジーを用いてさまざまな濃度で導入されたGFP陽性ヒトCD34+末梢血幹細胞(PBSC)の数が、導入後12日目で最大80%に達しました。
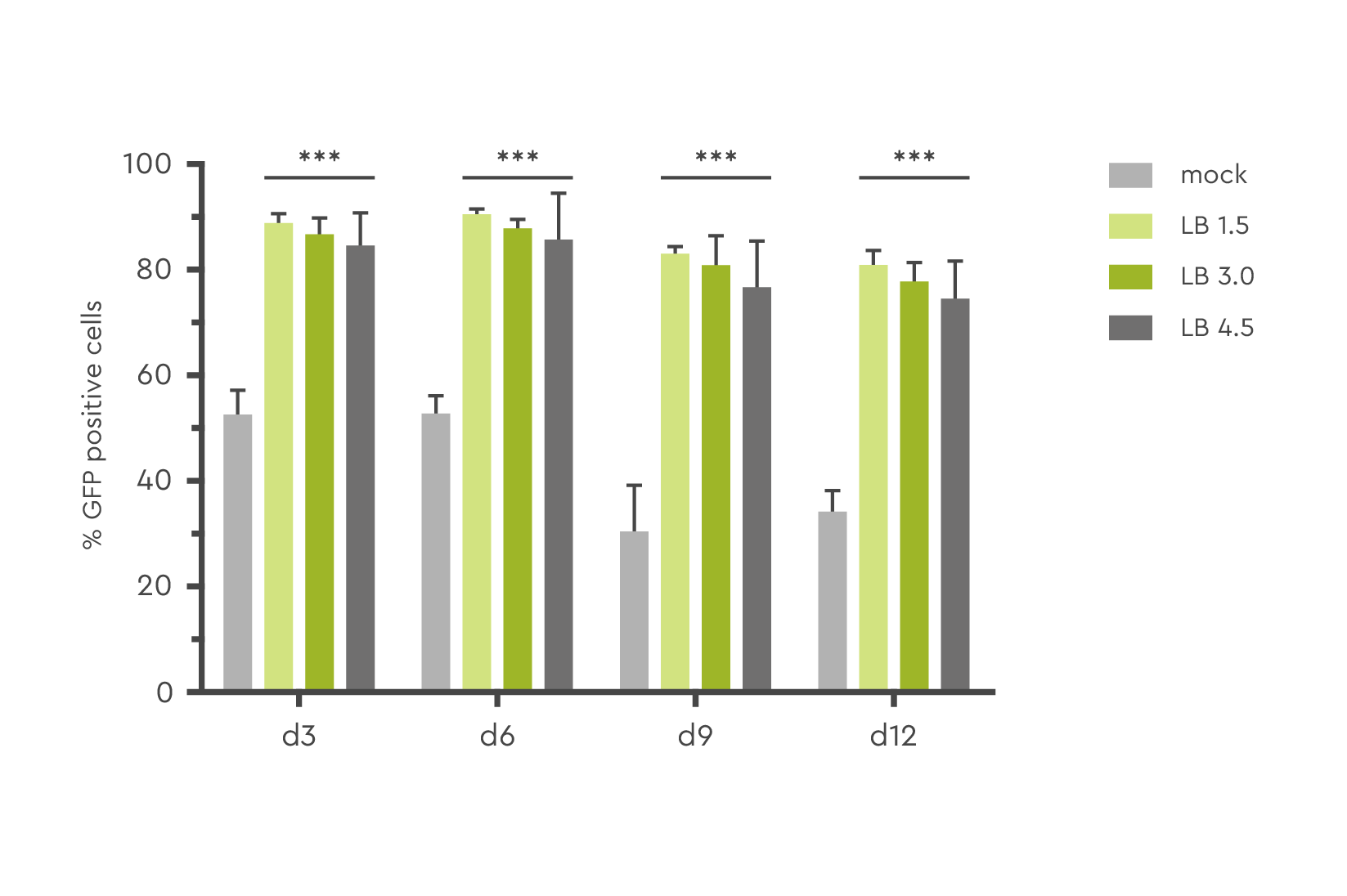
図1:さまざまな濃度のLentiBOOST形質導入エンハンサー(LB;1.5 mg/mL~4.5 mg/mL)ーを用いてレンチウイルス導入を行ったGFP陽性ヒトCD34+末梢血幹細胞(PBSC)の割合を、形質導入後3日目、6日目、9日目、12日目に測定しました。出典:Hauber et al., 2018
LentiBOOSTテクノロジーは、細胞あたりのベクターコピー数を調整・制御可能とし、EMA/FDAの安全性ガイドラインに沿った最適化を可能にします。これにより、様々な濃度を使用する際にも、治療用タンパク質の発現を促進することが可能となります。
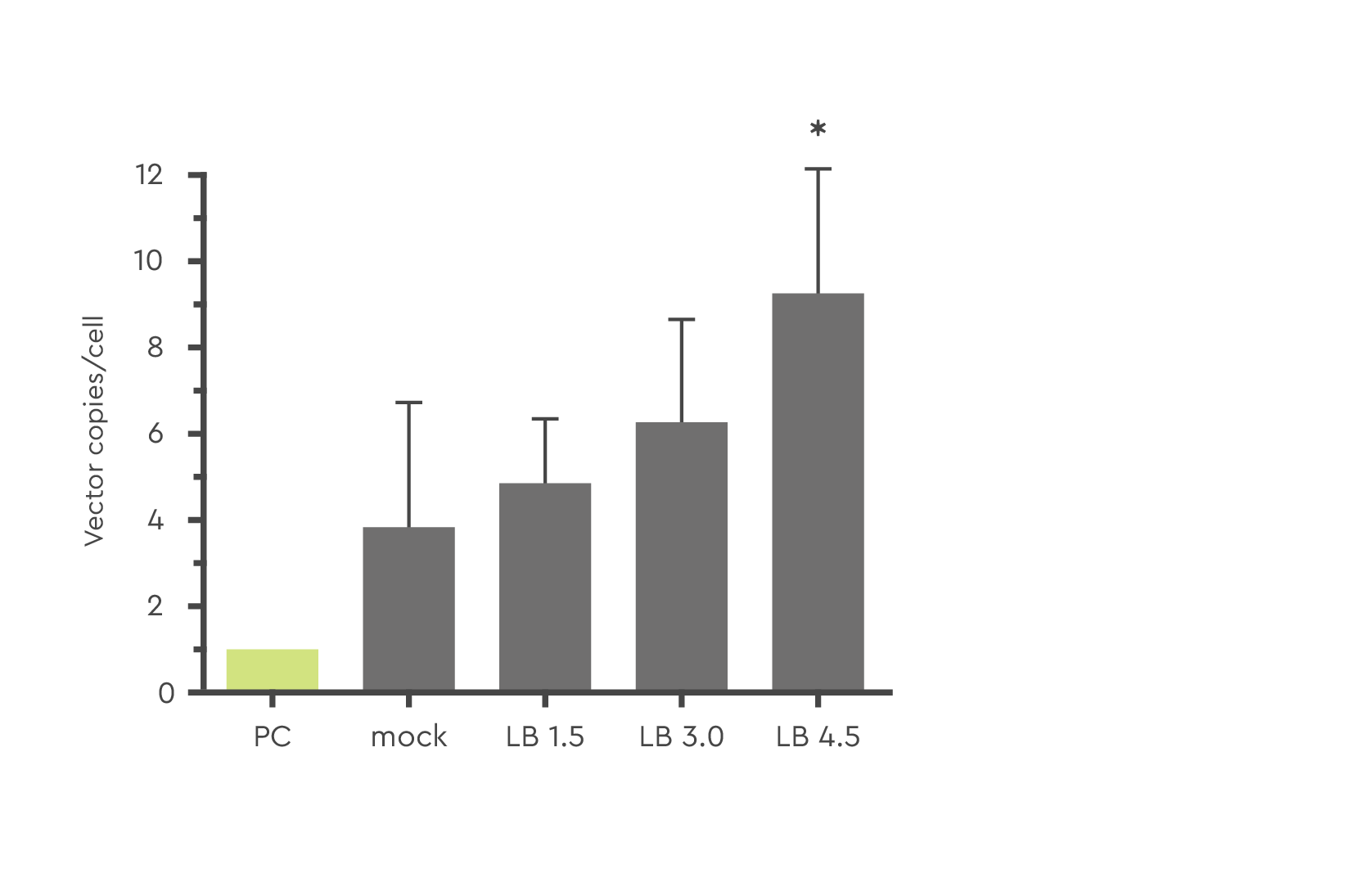
図2:ヒトCD34+末梢血幹細胞(PBSC)におけるLV-GFP(MOI=10)を用いたレンチウイルス導入効率に対する、LentiBOOST形質導入エンハンサー試薬の濃度変化(1.5~4.5 mg/mL)の影響を示します。星印(*)はp ≤ 0.05を示します。出典:Hauber et al., 2018
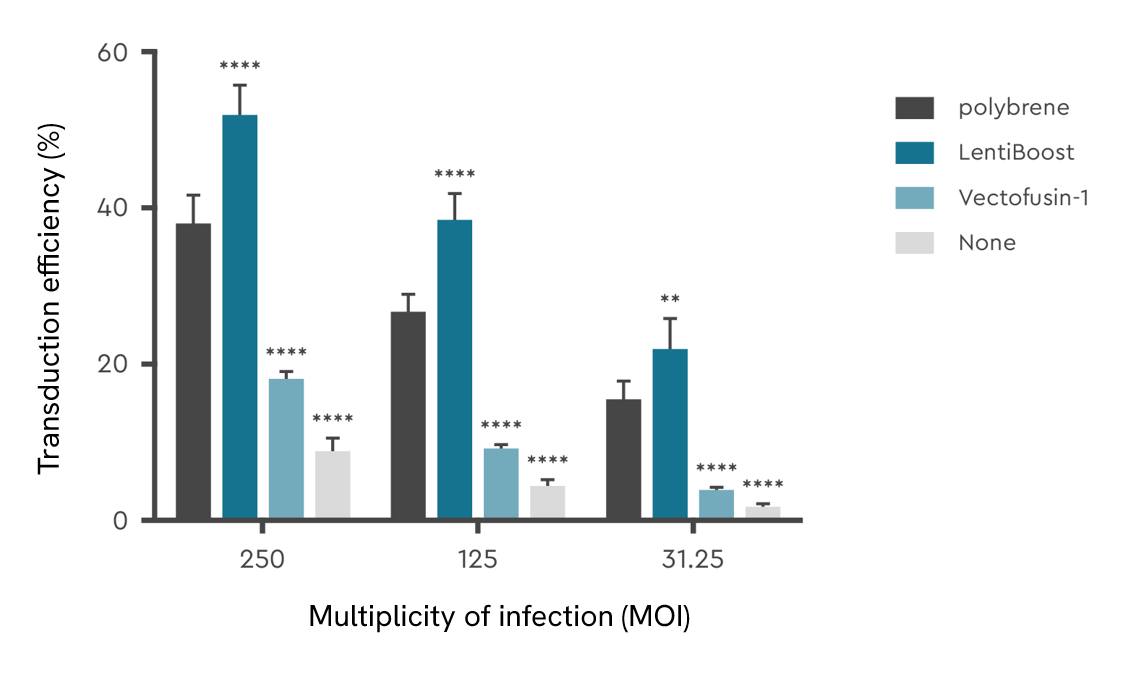
お客様によるLentiBOOSTテクノロジーと他社のレンチウイルス形質導入エンハンサーとの直接比較試験において、試験した3濃度範囲において、LentiBOOSTプラットフォームがヒトT細胞の導入に対して最も強い効果を示しました。最高感染多重度(MOI)では、LentiBOOSTプラットフォームの導入増強効果が最も顕著であり、増強剤を使用しないレンチウイルス導入と比較して約5倍の効率が確認されました。
幅広い細胞タイプで効果を発揮
LentiBOOSTレンチウイルス形質導入エンハンサーは、CD34+造血幹細胞(HSC)、間葉系幹細胞(MSC)、神経幹細胞、初代T細胞、導入が困難なマウスT細胞、NK細胞、線維芽細胞などの臨床的に重要な幅広い細胞タイプに適用可能です。この技術は、ex vivo遺伝子治療やCAR-T細胞療法の臨床用遺伝子導入プロトコルに最適です。
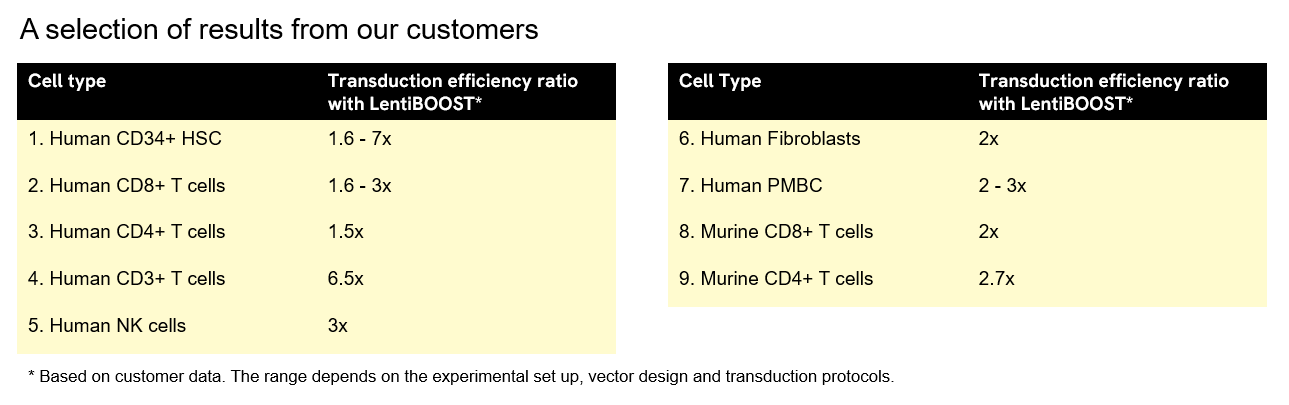
以下は、お客様データに基づくLentiBOOST形質導入エンハンサーを使用した場合における導入細胞数の増加率(単位倍増)を示しています。この範囲は、実験設定、ベクター設計、およびトランスダクションプロトコルによって異なります。
細胞毒性が観察されず、分化能を維持
レンチウイルスによる形質導入効率の最適化は、細胞の健全性や機能性を損なうことを代償として行ってはなりません。この点は様々な研究で評価されており、LentiBOOSTテクノロジーで処理された造血幹細胞(HSC)は、対照細胞と同等の生存率を示しました。
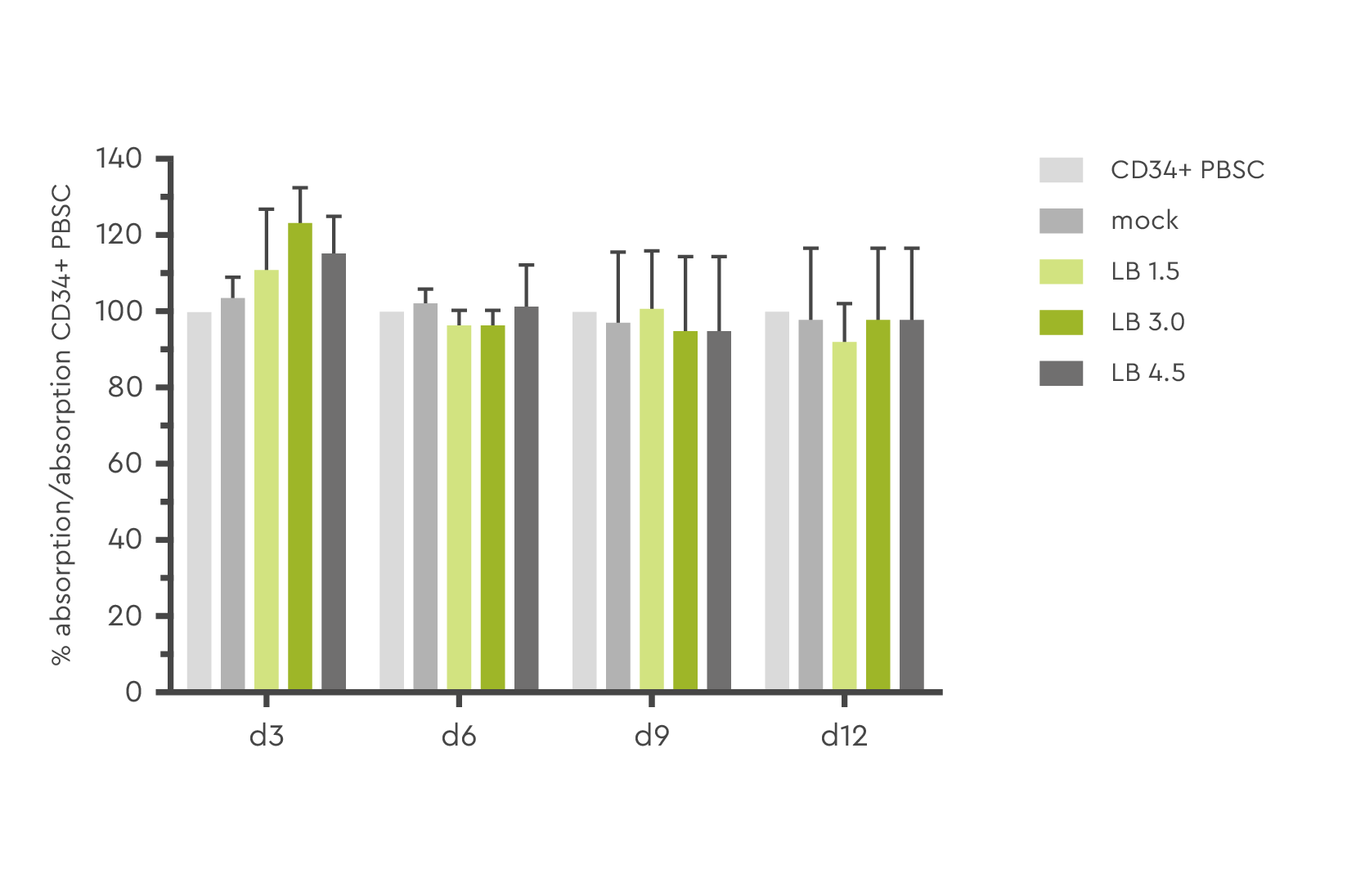
図3: トランスダクション後、3日目、6日目、9日目、12日目におけるMTTアッセイでの細胞生存率および毒性の測定。出典: Hauber et al., 2018
細胞健康状態と同様に重要なのが、細胞の分化能です。実験により、LentiBOOSTテクノロジーは造血幹細胞(HSC)が様々な造血系へ分化する能力に影響を与えないことが示されています(Hauber et al. 2018)。
優れた実績
LentiBOOST™テクノロジーは、40件以上の第I/II相および第III相臨床試験に採用され、承認済み細胞および遺伝子治療製品2品目に使用されています。また、世界中の600を超える学術機関および産業顧客に利用されています(2024年5月時点)。
LentiBOOSTプラットフォームの主なご利用事例をご紹介します:
- Mustang Bio (NCT01306019):MB-207プログラムは、X連鎖重症複合免疫不全患者の治療を目的としたレンチウイルス療法の評価を目的としています。臨床開発は第I/II相試験です(2021年末時点)。詳細はこちら
- カリフォルニア大学サンフランシスコ校 (NCT03538899):この研究の目的は、アルテミス欠損型重症複合免疫不全症患者の治療です。患者は自己の遺伝子導入幹細胞を投与し、DCLRE1C遺伝子の正常なコピーを導入することで免疫機能の機能を回復させます。この治療法は第I/II相試験で評価中です(2021年末時点)。詳細はこちら
- 国立アレルギー感染症研究所:同研究所のプログラムは、X連鎖重症複合免疫不全症(SCID-X1)の患者の治療を目的としています。患者のCD34+細胞にSCID-X1患者で障害されているILR2G遺伝子の正常なコピーを発現させるための遺伝子導入を行います。詳細はこちら
- オーチャード・セラピューティクス:このバイオ医薬品企業は、希少疾病の遺伝子治療製品に組み込むためにLentiBOOSTテクノロジーのライセンスを取得しました。詳細はこちら
- Cellectis:このバイオ医薬品企業は、同種CAR-T細胞の製造技術ポートフォリオを拡張するために、LentiBOOSTテクノロジーのライセンスを取得しました。詳細はこちら
- Beam Therapeutics:この米国企業は、独自の塩基編集技術を使用した次世代のCAR-T製品候補に適用するため、LentiBOOSTプラットフォームのライセンスを取得しました。。詳細はこちら
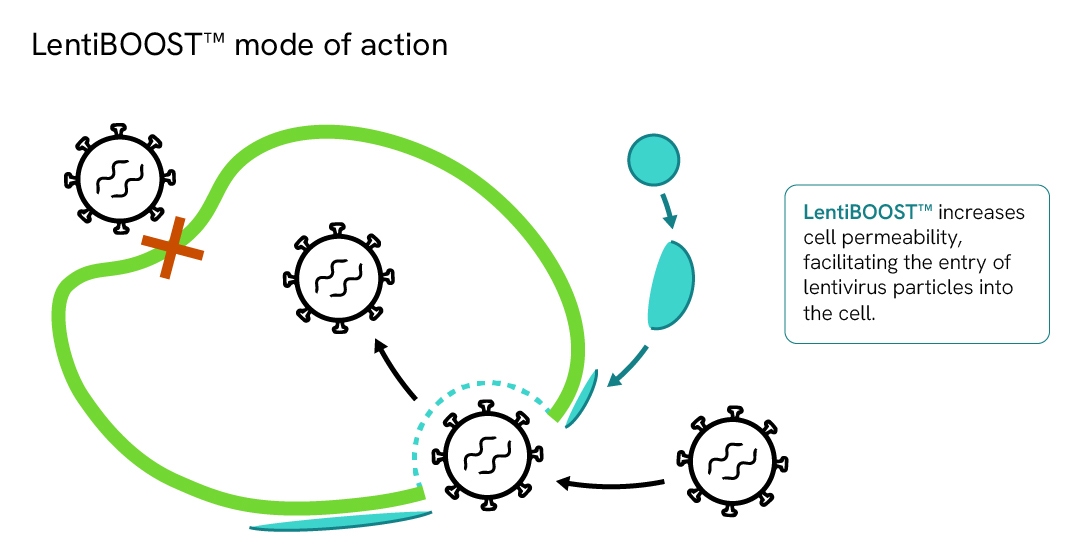
LentiBOOSTエンハンサーの作用機序
LentiBOOSTテクノロジーは、多様な細胞タイプにおけるレンチウイルスベクターの研究および臨床応用向けに開発された、極めて効果的で非細胞毒性のトランスダクションエンハンサーです。
本技術は、受容体に依存しない汎用的な製造補助剤として機能し、レンチウイルスと細胞膜の融合を促進し、ベクターコピー数を増加させ、より低いMOI(感染倍率)においてレンチウイルスのトランスダクション効率を大幅に改善します。
研究用LentiBOOST Pharma-Grade試薬
LentiBOOSTプラットフォームは、研究およびプロセス開発を目的として、世界中でPharma-Gradeでご提供しております。Revvityは、研究およびプロセス開発(大規模な実験を含む)向けに、LentiBOOST Pharma-Grade(non-GMP)材料を供給しております。LentiBOOST テクノロジー(Pharma-Grade )は、世界中の数百の研究室でご利用いただいております。
臨床使用のためのLentiBOOST GMP-Grade試薬
LentiBOOSTテクノロジーGMP-Gradeマテリアルは、臨床開発および商業段階における細胞治療向けに、臨床ライセンスまたは商業ライセンスのもとでご利用いただけます。
LentiBOOST形質導入エンハンサーは、臨床試験の反応率と全体的な成功率の向上に貢献します。当社のライセンシー様は、LentiBOOSTプラットフォームをプロセスに統合することで、製造の堅牢性を高め、臨床および商業用途の両方においてGMPマテリアルをご利用いただけます。
私たちは、特に商業段階において、信頼性と安全性を備えた供給網がいかに重要であるかを理解しています。そのため、規制要件に準拠したGMP条件下での自動充填・仕上げ工程を採用してます。当社の品質保証プロセスは、最高水準の基準への適合性とバッチ間差の少ない一貫性を保証します。LentiBOOSTテクノロジーGMPグレード製品には、FDAやEMAなどの規制当局向けに、CoA(分析証明書)および必要な全書類が付属します。
当社は、細胞治療の臨床承認を目指すライセンシーをパートナーとして密接に支援しています。臨床的成功を推進するため、お客様のニーズに応える個別化されたライセンスモデルをご提供しています。
Revvityでは主に2種類のライセンスを提供しています:
- 商業ライセンス: 細胞治療プログラムを開発および商業化している企業向け。
- 研究者主導の臨床試験向けアカデミックライセンス:新たな細胞治療を開発する先駆者を支援するため、Revvityは非営利研究機関が臨床試験の規制当局に対する責任を負う場合に限り、アカデミックパートナー様向けにLentiBOOSTテクノロジーのアカデミックライセンスを提供し、臨床プログラムでのご利用を可能にしております。
必要なLentiBOOSTエンハンサーの量は?
材料は100mg/mLの濃度で供給されます。ほとんどの用途では、LentiBOOSTレンチウイルス形質導入エンハンサー試薬は1:100から1:400の希釈で使用されます。