
pHSense Eu Anti-FLAG, 10 x 96 wells
| Feature | Specification |
|---|---|
| Application | 内部化 |
| Sample Volume | 50 µL |



Product information
Overview
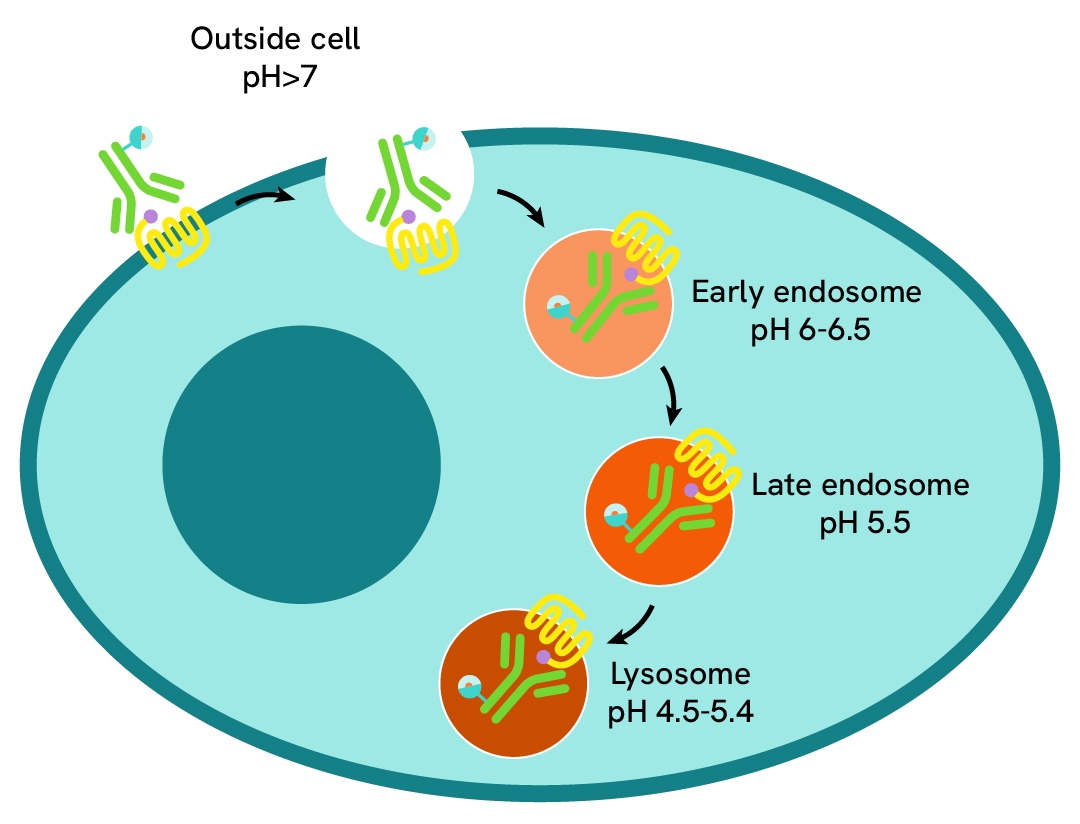
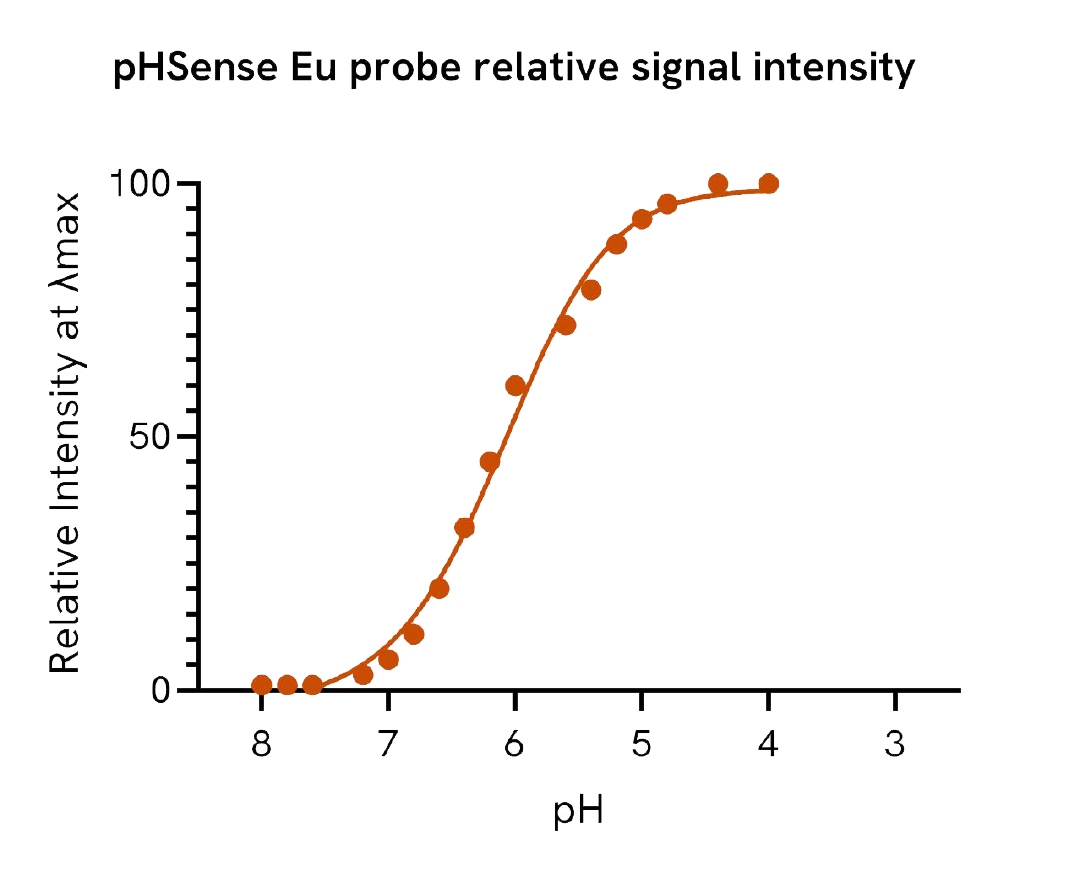
pHSense™ Eu Anti-FLAG is non cell permeant, and is labeled with a pH sensitive europium complex. This Monoclonal Anti-FLAG antibody is designed to monitor receptor and membrane protein internalization when tagged with the FLAG® sequence (DYKDDDDK) on the extracellular part. The antibody recognizes the FLAG® sequence fused at the N-terminus, intra or C-terminus and remains minimally fluorescent at neutral extracellular pH (≥7). Once internalized, the pHSense™ Eu Anti-FLAG encounters increasingly acidic compartments such as early and late endosomes and lysosomes, where the europium signal becomes progressively stronger. This new pH sensitive europium anti-FLAG is compatible with a time-resolved fluorescence (TRF) detection, effectively eliminating most fluorescence background and significantly enhancing the signal-to-background ratio. Its unique photophysical properties enable simple and robust no-wash detection of receptor-mediated endocytosis in plate-based assays with live-cells.
How it works
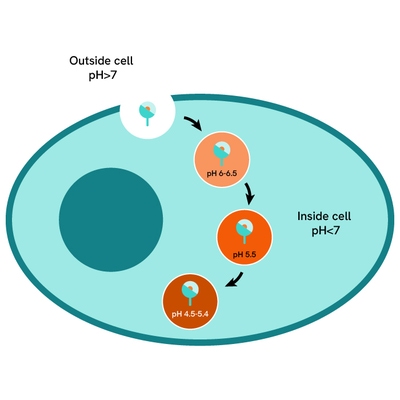
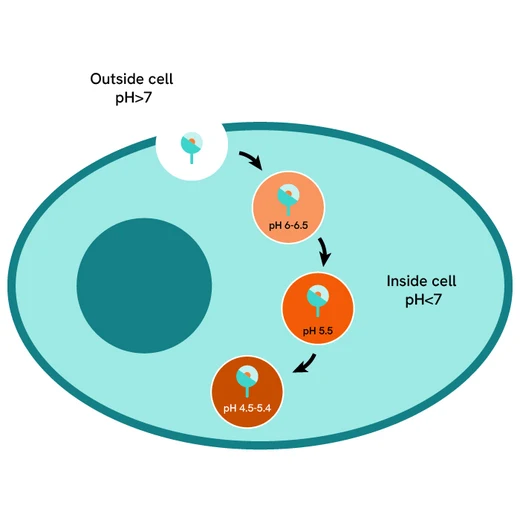
pHSense Eu-Anti-FLAG assay principle
pHSense Eu Anti-FLAG is non cell permeant, and is labeled with a pH sensitive europium complex. This Monoclonal Anti-FLAG antibody is designed to monitor receptor and membrane protein internalization when tagged with the FLAG® sequence. The antibody recognizes the FLAG sequence and remains minimally fluorescent at neutral extracellular pH (≥7). Once internalized, the pHSense Eu Anti-FLAG encounters increasingly acidic compartments such as early and late endosomes and lysosomes, where the europium fluorescent signal becomes progressively stronger.

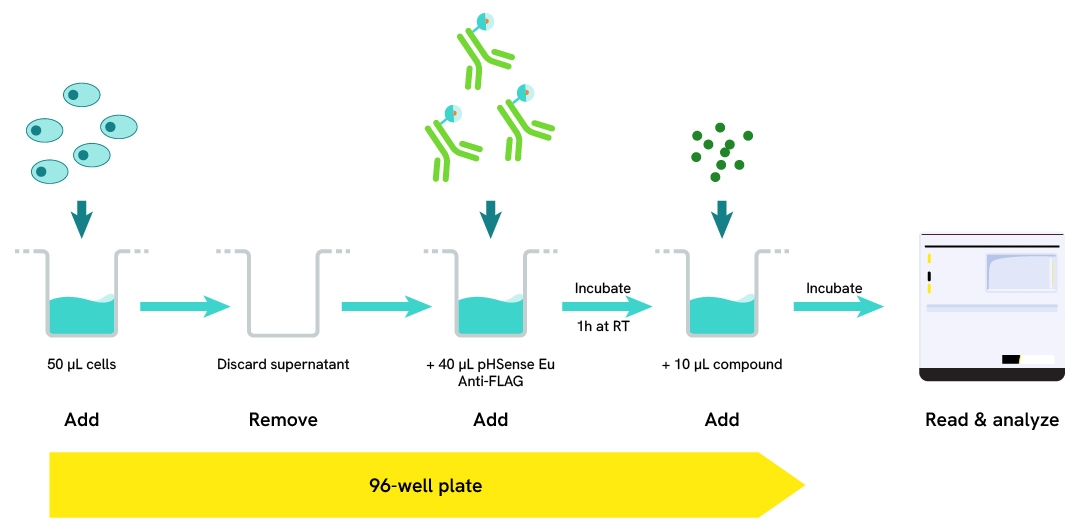
pHSense Eu Anti-FLAG Assay Protocol
The assay begins by culturing cells in a 96-well plate. The pHSense Eu Anti-FLAG is then added to the cells and incubated for 1 hour at room temperature. Following this incubation, cells are stimulated with a pharmacological compound. Fluorescence is then measured either kinetically or at endpoint using an HTRF-compatible plate reader.
Assay validation
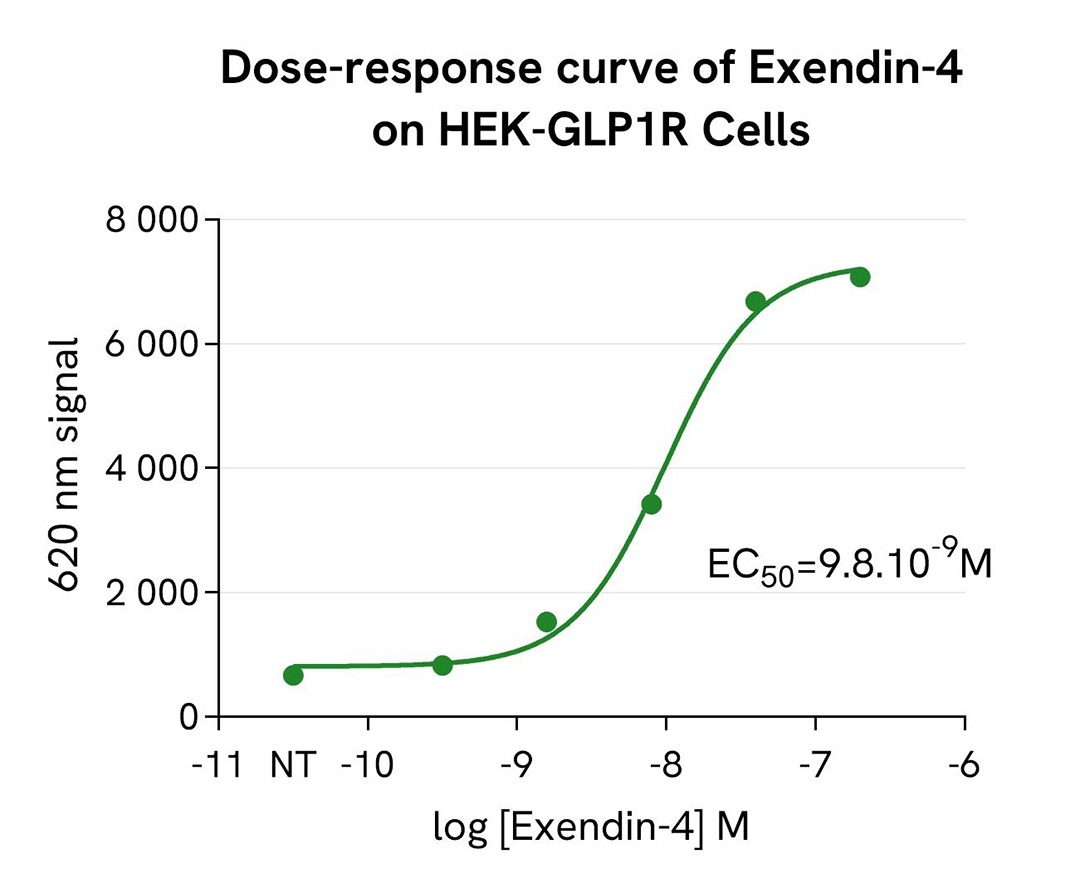
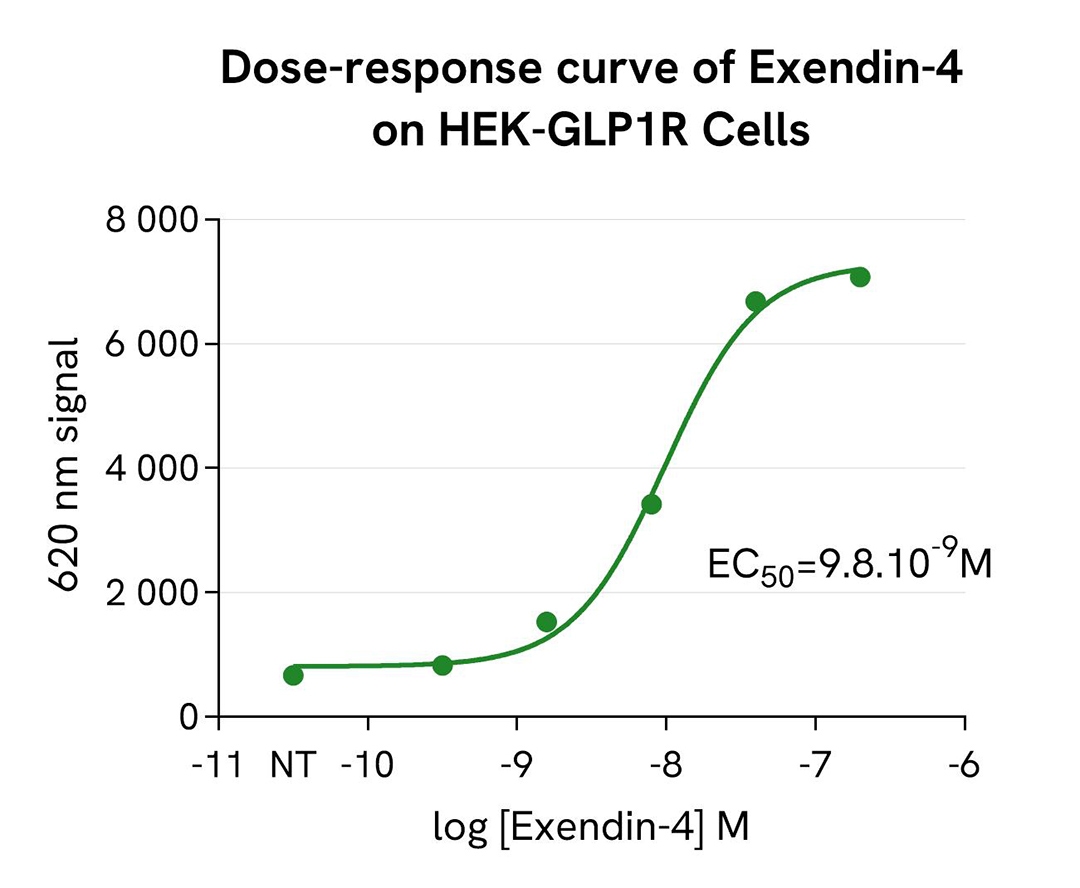
pHSense-Based Detection of GLP1R Internalization Following Exendin-4 Stimulation
Tag-Lite® GLP1R cells (stable cell line, Part #: C1SU1GLP1, Revvity) were seeded in a 96-well white, culture-treated plate at a density of 80,000 cells per well in complete culture medium and incubated overnight at 37°C with 5% CO₂. After cell supernatant removal, 40 µL of the pHSense Eu Anti-FLAG diluted in cell culture medium (DMEM +10%FBS) were added to the cells, followed by a 1 hour incubation at room temperature.
Exendin-4, a GLP1R agonist, was serially diluted in cell culture medium, and 10 µL of each dilution were added to the wells. After incubation at 37°C, the signal was recorded using an HTRF-compatible plate reader. Results show Exendin-4 induced a dose-dependent increase in signal, with an EC₅₀ value consistent with values described in the scientific literature.
In parallel, cells were pre-incubated with 10 µM of the GLP1R antagonist Exendin 9-39 for 30 minutes at 37°C prior to the addition of Exendin-4. Following a 20 min incubation at 37°C, the signal was recorded. In presence of Exendin 9-39 the signal decreased to a level close to the constitutive internalization, which suggests effective inhibition of agonist induced GLP1R internalization.
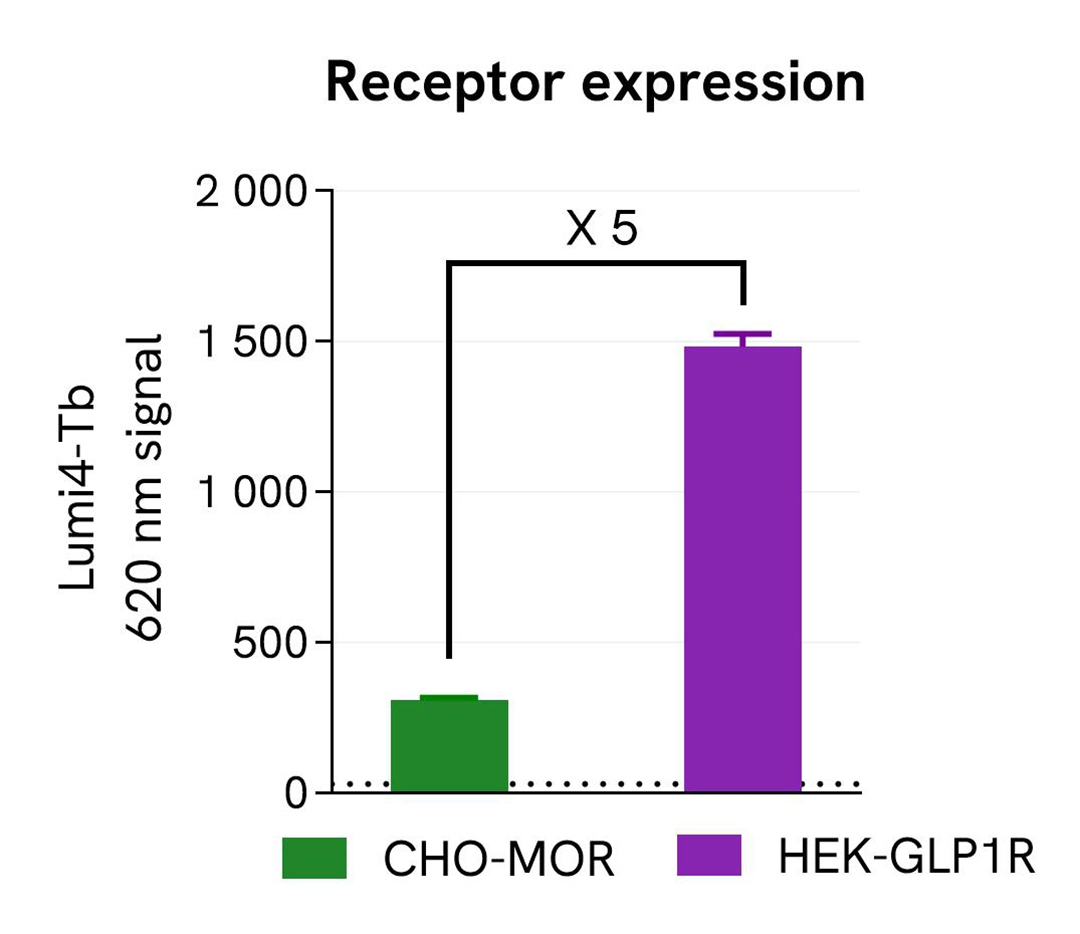
Validation of pHSense Eu Anti-FLAG assay in cell lines expressing different receptor levels
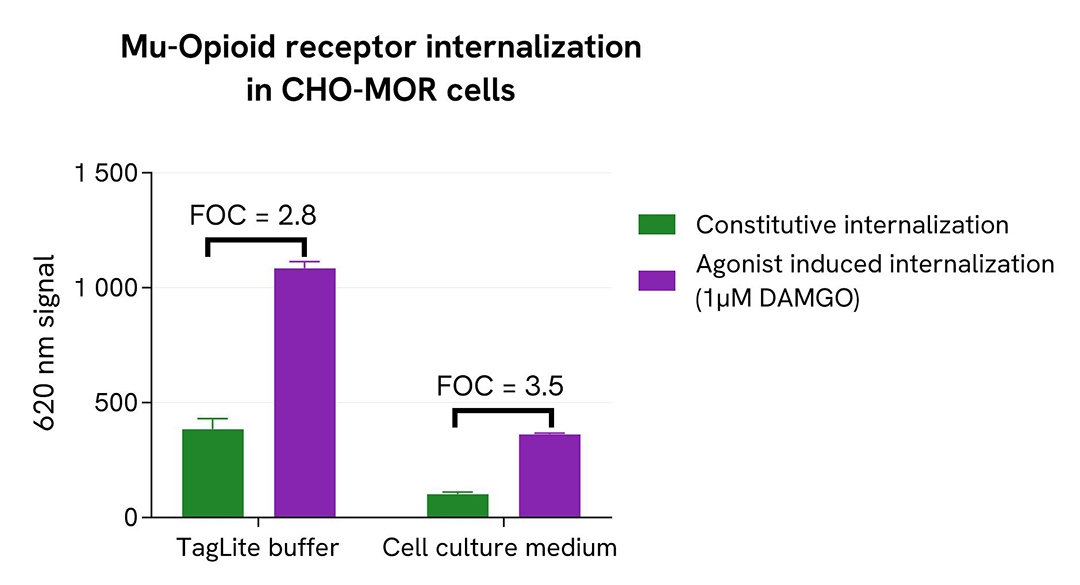
The pHSense Eu Anti-FLAG was tested on 2 different stable cell lines expressing various level of receptors at the membrane: Tag-Lite® GLP1R cells (HEK-GLP1R) and Tag-lite® Mu opioid cells (CHO-MOR).
The relative levels of receptor expression levels were determined using the Tag-lite SNAP-Lumi4-Tb Labeling Reagent (Part#: SSNPTBC, Revvity). Cells were seeded in a 96-well black culture-treated plate at a density of 100,000 cells per well in complete culture medium. After overnight incubation at 37°C with 5% CO₂, the supernatant was removed and 100 µL of 100 nM SNAP-Lumi4-Tb Labeling Reagent were added to the cells, followed by a 1h incubation at 37°C with 5% CO₂. Cells were washed 3 times with Tag-lite SNAP/CLIP Labeling Medium 1X (Part#: LABMED, Revvity) before the addition of 100 µL of the same medium. The 620 nm signal was recorded using an HTRF-compatible plate reader. Results showed Tag-Lite® GLP1R cells exhibit a 5-times higher expression level than Tag-lite® Mu opioid cells.
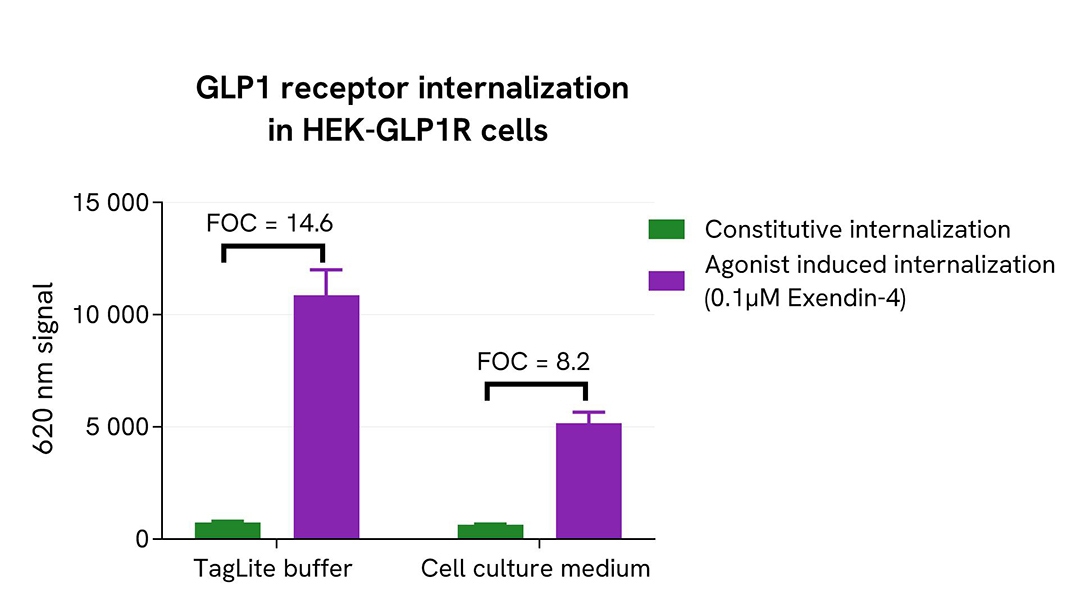
To run the pHSense Eu Anti-FLAG assay, Tag-Lite® GLP1R and Tag-lite® Mu opioid stable cell lines were seeded in a 96-well white culture-treated plate at a density of 80,000 cells per well in complete culture medium, and then incubated overnight at 37°C with 5% CO2.
After cell supernatant removal, 40 µL of the pHSense Eu Anti-FLAG diluted in cell culture medium were added to the cells and incubated for 1 hour at room temperature. Exendin-4, a GLP1R agonist, and DAMGO, a Mu opioid agonist, were diluted in cell culture medium, and 10 µL of agonists or cell culture medium - as constitutive internalization condition - were added to the wells. After incubation at 37°C, the signal was recorded using an HTRF-compatible plate reader.
In parallel, the same assay was performed with the pHSense Eu Anti-FLAG and receptor agonists prepared in Tag-lite SNAP/CLIP Labeling Medium as physiological buffer.
As demonstrated here, the pHSense Eu Anti-FLAG allows the detection of agonist induced and constitutive internalization in cell lines with different receptor expression levels, and is compatible with Tag-lite SNAP/CLIP Labeling Medium as physiological buffer or cell culture medium.
Specifications
| Application |
Internalization
|
|---|---|
| Brand |
pHSense
|
| Detection Modality |
pH sensitive dye
|
| Product Group |
Fluorescent Reagent
|
| Sample Volume |
50 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target |
FLAG
|
| Target Class |
Cell surface proteins, antibodies, ADCs
|
| Technology |
TRF
|
| Unit Size |
10 x 96 wells
|
Resources
Are you looking for resources, click on the resource type to explore further.
pHSense™ probes are plate reader-compatible reagents with time-resolved fluorescence (TRF) readout specifically developed for...
Discover pHSense™ probes – plate reader-compatible reagents with time-resolved fluorescence (TRF) readout, specifically developed...


How can we help you?
We are here to answer your questions.