
AlphaLISA SureFire Ultra Human and Mouse Phospho-TYK2 (Tyr1054/1055) Detection Kit, 100 Assay Points

 View All
View All






| Feature | Specification |
|---|---|
| Application | 細胞シグナル伝達 |
| Sample Volume | 30 µL |












Product information
Overview
Tyrosine kinase 2 (TYK2) is a member of the JAK1, JAK2, and JAK3 family of non-receptor Janus tyrosine kinases. Phosphorylation of TYK2 is induced by a broad range of cytokines and growth factors (IL12, IL23, and interferons) bound to their receptors. The main substrates, STATs, and cytokine receptors are among the other targets that the activated TYK2 goes on to phosphorylate. Cell division, migration, apoptosis, and proliferation are all accelerated by JAKs/STATs signaling. An appealing aim for anti-inflammatory treatments is to modify JAK/STATs signaling to lessen cytokine-induced pro-inflammatory responses.
The AlphaLISA SureFire Ultra Human and Mouse Phospho-TYK2 (Tyr1054/1055) Detection Kit is a sandwich immunoassay for the quantitative detection of phospho-TYK2 (Tyr1054/1055) in cellular lysates, using Alpha Technology.
Formats:
- The HV (high volume) kit contains reagents to run 100 wells in 96-well format, using a 60 μL reaction volume.
- The 500-point kit contains enough reagents to run 500 wells in 384-well format, using a 20 μL reaction volume.
- The 10,000-point kit contains enough reagents to run 10,000 wells in 384-well format, using a 20 μL reaction volume.
- The 50,000-point kit contains enough reagents to run 50,000 wells in 384-well format, using a 20 μL reaction volume.
AlphaLISA SureFire Ultra kits are compatible with:
- Cell and tissue lysates
- Antibody modulators
- Biotherapeutic antibodies
Alpha SureFire kits can be used for:
- Cellular kinase assays
- Receptor activation studies
- Screening
How it works
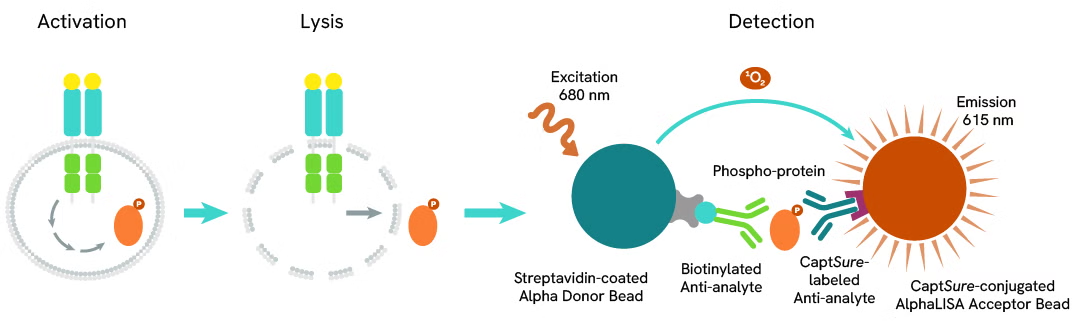
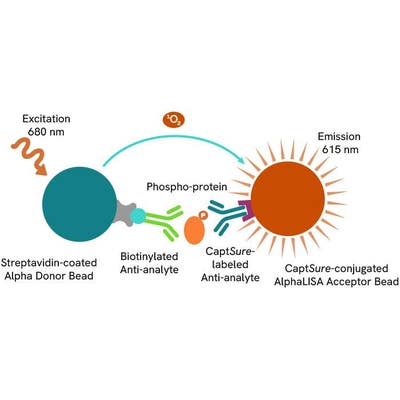
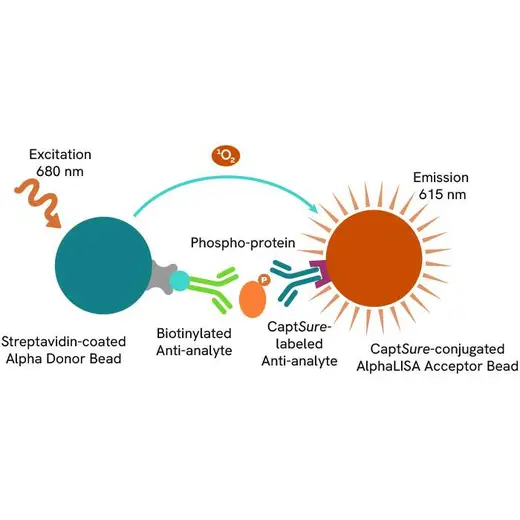
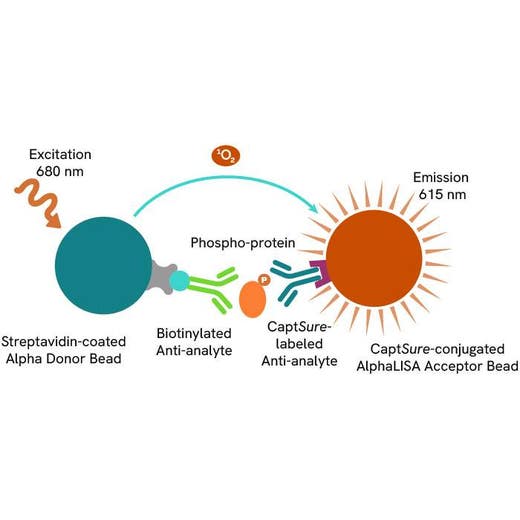
Phospho-AlphaLISA SureFire Ultra assay principle
The Phospho-AlphaLISA SureFire Ultra assay measures a protein target when phosphorylated at a specific residue.
The assay uses two antibodies which recognize the phospho epitope and a distal epitope on the targeted protein. AlphaLISA assays require two bead types: Acceptor and Donor beads. Acceptor beads are coated with a proprietary CaptSure™ agent to specifically immobilize the assay specific antibody, labeled with a CaptSure tag. Donor beads are coated with streptavidin to capture one of the detection antibodies, which is biotinylated. In the presence of phosphorylated protein, the two antibodies bring the Donor and Acceptor beads in close proximity whereby the singlet oxygen transfers energy to excite the Acceptor bead, allowing the generation of a luminescent Alpha signal. The amount of light emission is directly proportional to the quantity of phosphoprotein present in the sample.

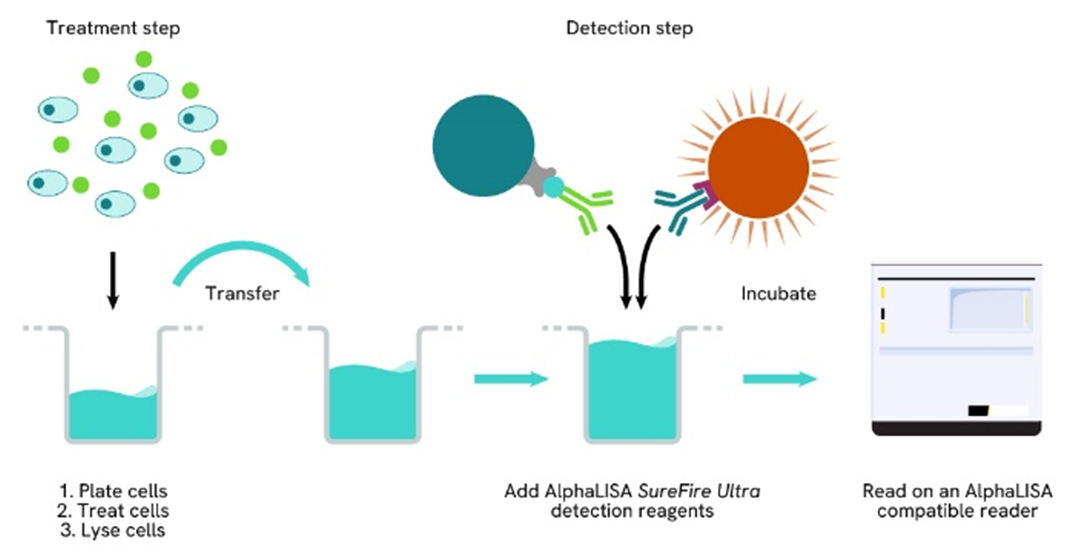
Phospho-AlphaLISA SureFire Ultra two-plate assay protocol
The two-plate protocol involves culturing and treating the cells in a 96-well plate before lysis, then transferring lysates into a 384-well OptiPlate™ plate before the addition of Phospho-AlphaLISA SureFire Ultra detection reagents. This protocol permits the cells viability and confluence to be monitored. In addition, lysates from a single well can be used to measure multiple targets.
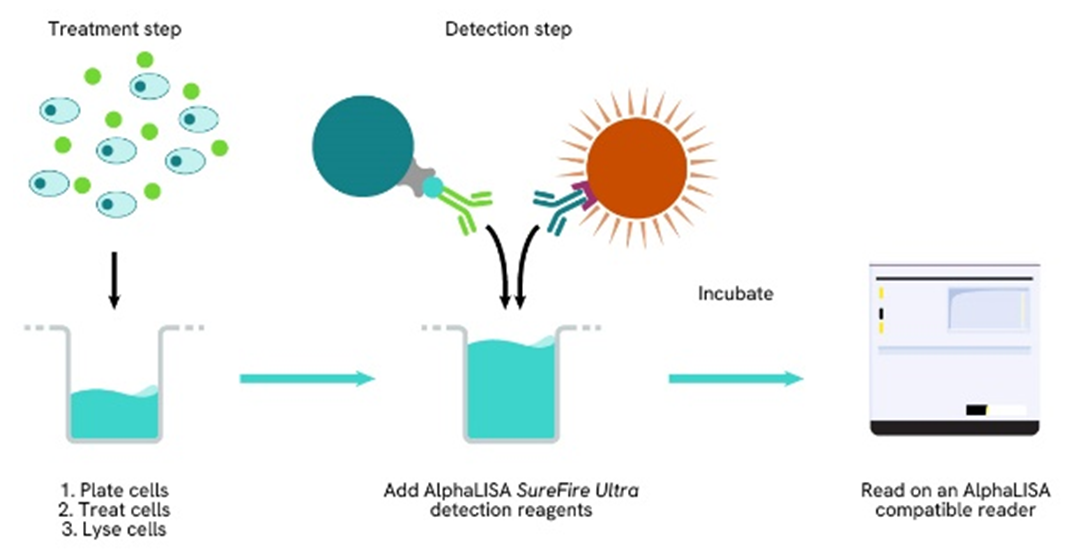
Phospho-AlphaLISA SureFire Ultra one-plate assay protocol
Detection of Phosphorylated target protein with AlphaLISA SureFire Ultra reagents can be performed in a single plate used for culturing, treatment, and lysis. No washing steps are required. This HTS designed protocol allows for miniaturization while maintaining AlphaLISA SureFire Ultra quality.
Assay validation
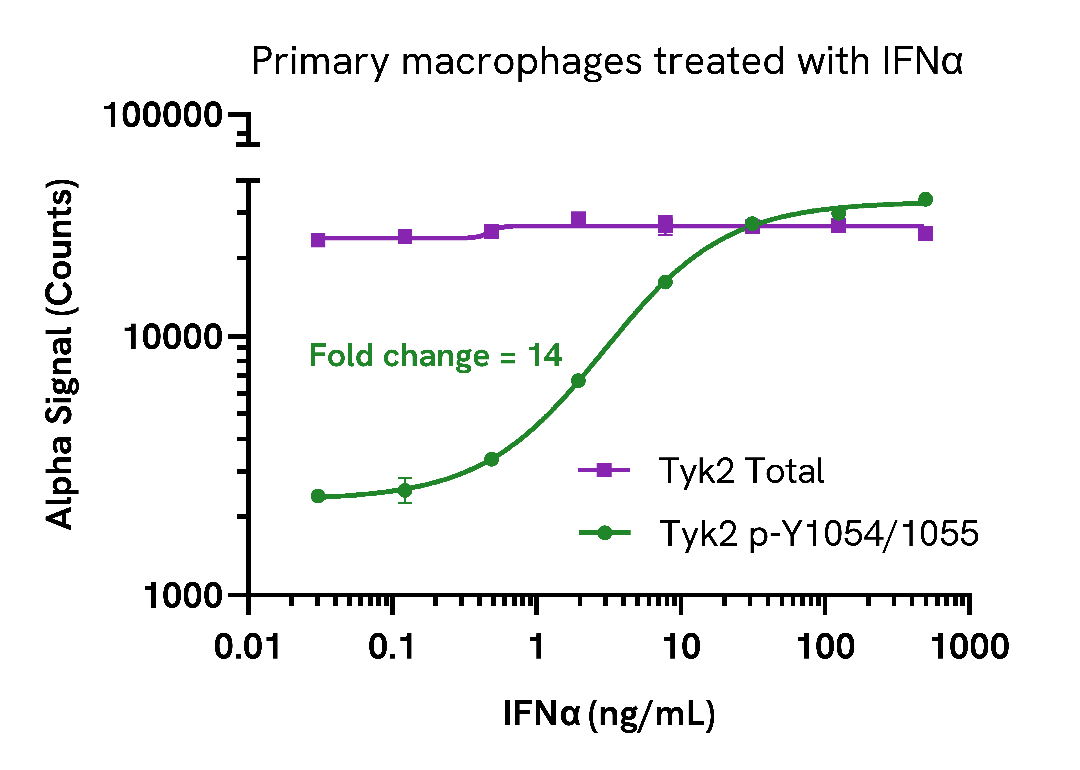
Induction of TYK2 (Tyr1054/1055) phosphorylation in primary cells
PBMCs were isolated from healthy donors and cultured for 6 days in complete DMEM containing 20 ng/mL M-CSF to differentiate them into macrophages. Macrophages were seeded in a 96-well plate (40,000 cells/well) in complete DMEM, and incubated overnight at 37°C, 5% CO2. Cells were starved for 2 hours and then treated with the indicated concentrations of IFNα for 20 minutes.
After treatment, the cells were lysed with 50 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Phospho (Tyr1045/1055) and Total TYK2 levels were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 8,000 cells) was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings
As expected, IFNα triggered a dose-dependent increase in the levels of Phospho (Tyr1054/1055) TYK2 while Total levels remained unchanged.
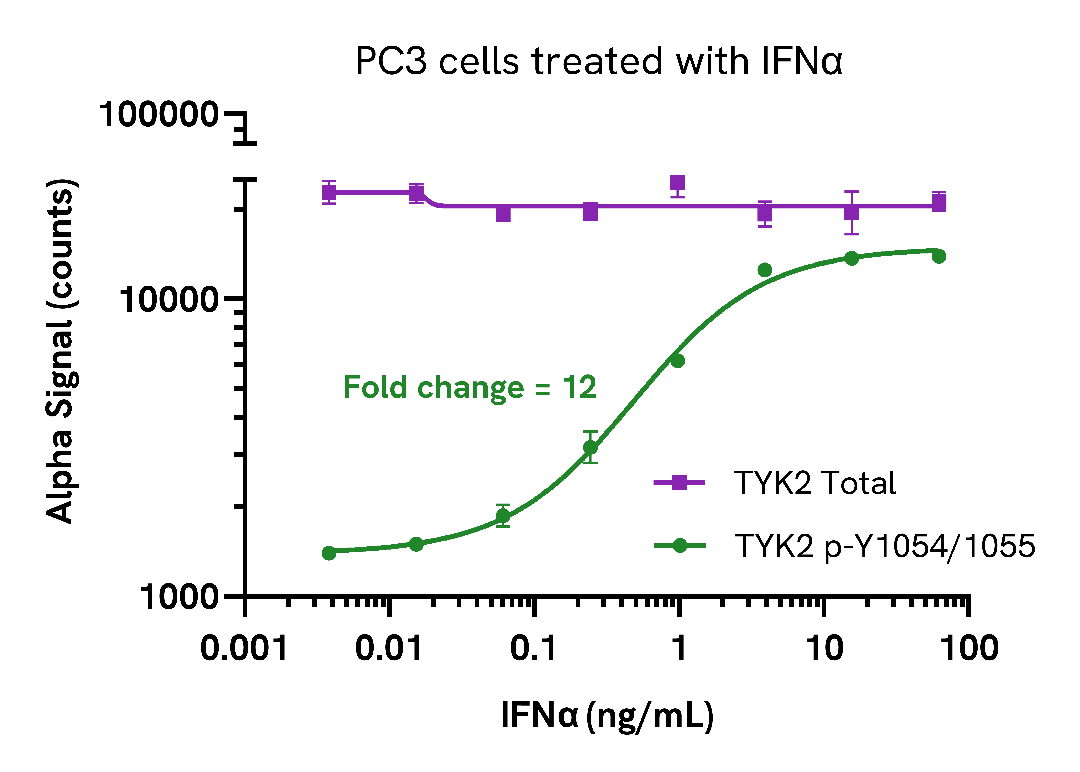
Induction of Phospho TYK2 (Tyr1054/1055) in endogenous cell models
PC3 cells were seeded in a 96-well plate (40,000 cells/well) in complete medium, and incubated for 24 hours at 37°C, 5% CO2. The cells were treated with increasing concentrations of IFNα for 15 minutes.
After treatment, the cells were washed with HBSS and lysed with 100 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Phospho (Tyr1054/1055) and Total TYK2 levels were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 4,000 cells) was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings
As expected, IFNα triggered a dose-dependent increase in the levels of Phospho (Tyr1054/1055) TYK2 while Total levels remained unchanged.
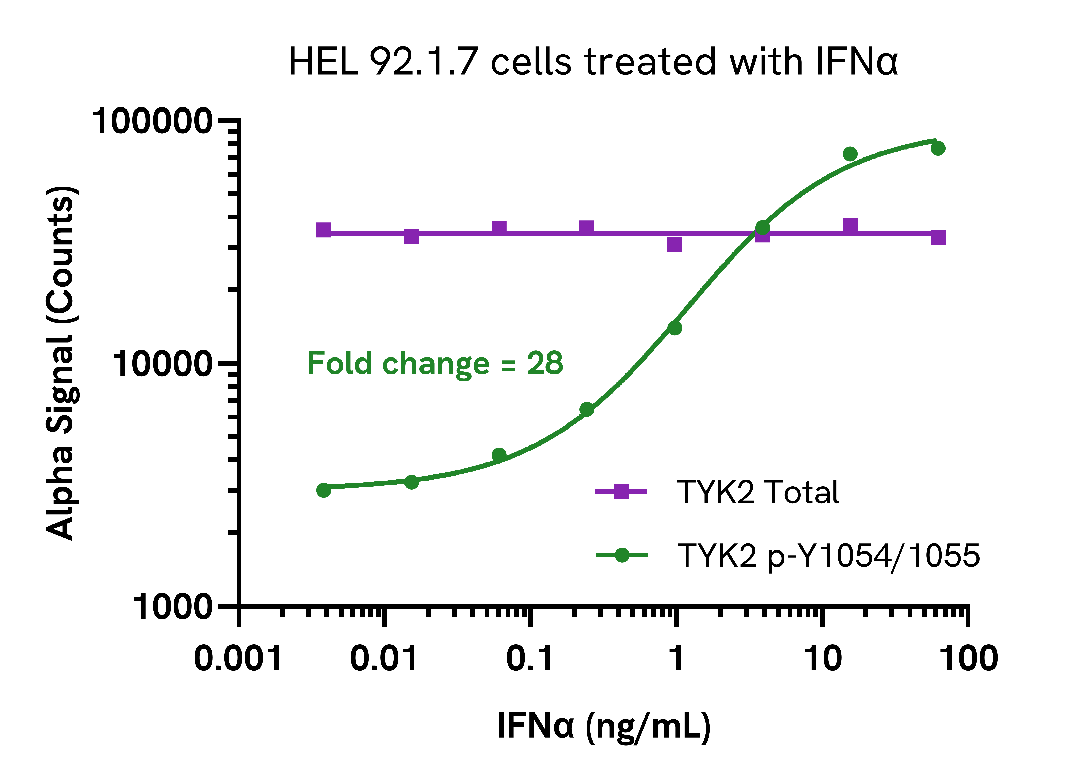
HEL 92.1.7 cells were seeded in a 96-well plate (500,000 cells/well) in HBSS + 0.1% BSA and treated with increasing concentrations of IFNα for 15 minutes.
After treatment, the cells were lysed with the addition of 50 µL of 5X Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Phospho (Tyr1054/1055) and Total TYK2 levels were evaluated using respective AlphaLISA SureFire Ultra assays. For the detection step, 10 µL of cell lysate (approximately 20,000 cells) was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings
As expected, IFNα triggered a dose-dependent increase in the levels of Phospho (Tyr1054/1055) TYK2 while Total levels remained unchanged.
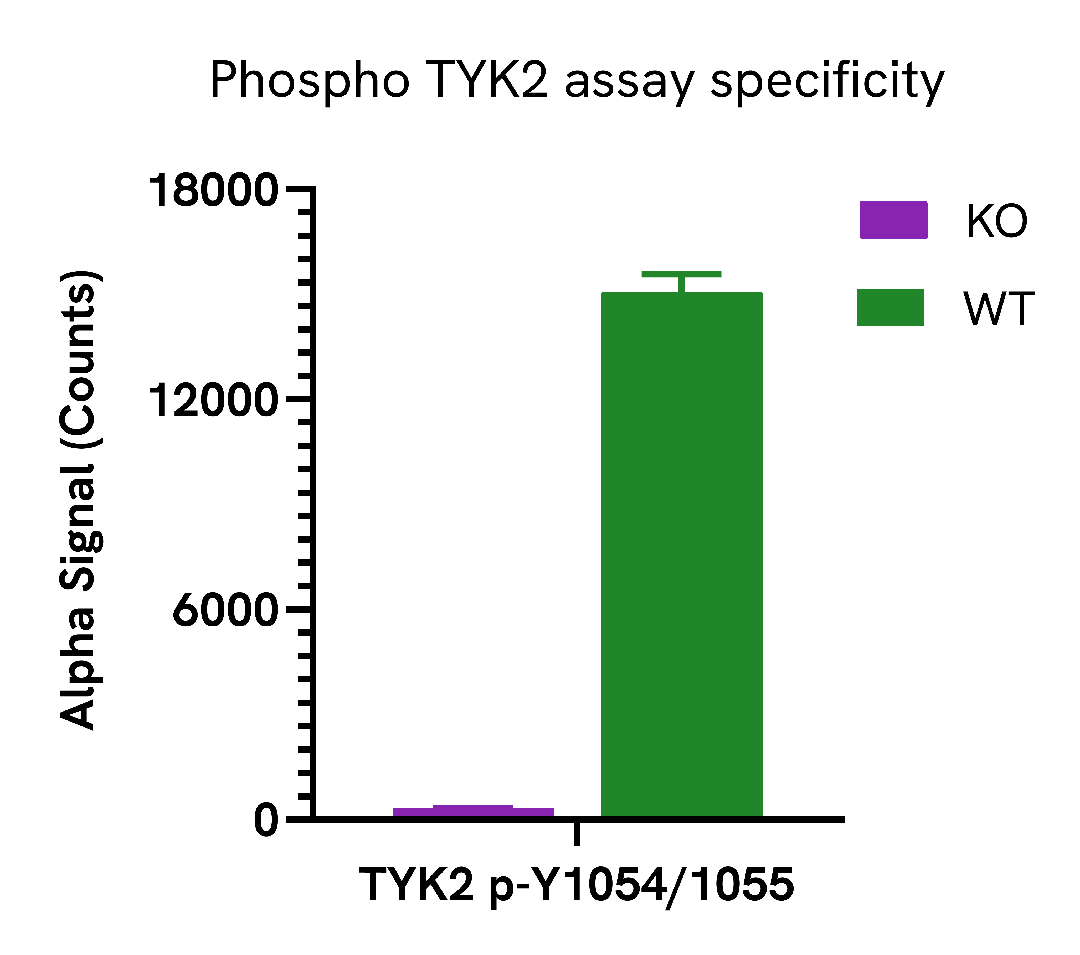
Assay specificity/selectivity
Specificity of Phospho TYK2 (Tyr1054/1055) assay
TYK2 (Tyr1054/1055) levels were assessed in Wild Type (WT) and TYK2 knockout (KO) HEK293T cells. TYK2 KO cells (Abcam ab266730) and WT HEK293T cells were seeded in a 96-well plate (40,000 cells/well) in complete medium, and incubated overnight at 37°C, 5% CO2. The cells were treated with 50 ng/mL IFNα for 10 minutes.
After treatment, the cells were lysed with 200 µL of Lysis Buffer for 10 minutes at RT with shaking (350 rpm). Phospho TYK2 (Tyr1054/1055) levels were evaluated by AlphaLISA SureFire Ultra. For the detection step, 10 µL of cell lysate (approximately 2,000 cells) was transferred into a 384-well white OptiPlate, followed by 5 µL of Acceptor mix and incubated for 1 hour at RT. Finally, 5 µL of Donor mix was then added to each well and incubated for 1 hour at RT in the dark. The plate was read on an Envision using standard AlphaLISA settings.
As expected, TYK2 (Tyr1054/1055) was detected in the WT HEK293T cells treated with IFNα but not in the TYK2 KO cell line.
Specifications
| Application |
Cell Signaling
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
AlphaLISA SureFire Ultra
|
| Detection Modality |
Alpha
|
| Lysis Buffer Compatibility |
Lysis Buffer
|
| Molecular Modification |
Phosphorylation
|
| Product Group |
Kit
|
| Sample Volume |
30 µL
|
| Shipping Conditions |
Shipped in Blue Ice
|
| Target |
TYK2
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
Mouse
|
| Technology |
Alpha
|
| Therapeutic Area |
Inflammation
|
| Unit Size |
100 assay points
|
Video gallery






Resources
Are you looking for resources, click on the resource type to explore further.
The definitive guide for setting up a successful AlphaLISA SureFire Ultra assay
Several biological processes are regulated by...


How can we help you?
We are here to answer your questions.