
Improving the efficiency of AAV characterization with LabChip technology webinar

Watch our on-demand webinar, AAV Characterization using LabChip Technology, to explore an innovative, reliable workflow developed to propel the efficiency of AAV characterization using a microfluidic electrophoresis platform.
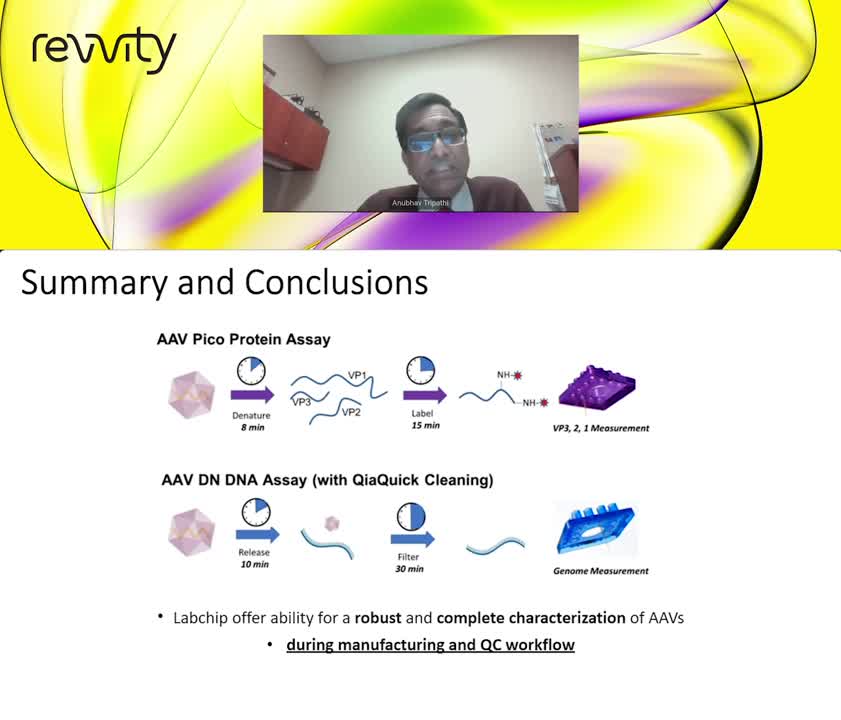
Adeno-associated virus (AAV) particles are intensively being investigated for therapeutic use in gene therapy due to low immunogenicity and ability for long-term gene expression in vivo. During development, testing strategies for the viral protein stoichiometry, capsid product purity, and ssDNA loading are used to monitor and assess AAV efficacy. The loading efficacy is observed as the percentage of capsids containing ssDNA (full capsids). While transmission microscopy (TEM) and analytical ultracentrifugation (AUC) measurements deliver reliable empty/full measurements, these methods have high cost and time requirements. As such, there is a need for a reliable, high-throughput platform characterization system for empty/full measurement that requires low AAV volume input.
In the first edition of this AAV characterization series, Dr. Tripathi will describe in detail how to characterize AAV particles and the critical quality attributes important to biopharma labs. We will detail existing analytical tools and unveil the LabChip microfluidic electrophoresis system which enables fast and reliable AAV characterization.
He discusses how this microfluidic capillary electrophoresis method measures AAV capsid proteins and ssDNA of samples alongside a well characterized AAV standard. Capsid protein and ssDNA area measurements are mathematically analyzed to determine the percentage of full AAV samples. This microfluidic system with an AAV standard referencing method enables microliter sized samples to be analyzed in ~1 minute per sample and is concentration independent. Measurement and calculation of empty:full percentage of AAV8 samples with concordance to the expected values (within 12% of target) was achieved for sample mixtures of full and empty reference AAV particles. The LabChip GXII Touch system provides a complete method for analysis of AAV capsids that improved the efficiency of gene therapy research and development.
For research use only. Not for use in diagnostic procedures.
To view the full content please answer a few questions





























