

End-to-end solutions for Companion Diagnostics (CDx): From assay development to regulatory affairs
Join us to an insightful webinar on the critical role of Companion Diagnostics (CDx) in the future of personalized medicine. As the demand for tailored therapeutic approaches grows, the need for precise and reliable diagnostics becomes paramount. Our webinar, titled "End-to-End Solutions for Companion Diagnostics (CDx): From Assay Development to Regulatory Affairs," will delve into the comprehensive process of developing and implementing CDx solutions, highlighting how Revvity Omics supports every step of this journey.
During this webinar, our experts will cover the entire CDx lifecycle, starting with assay development. You'll gain a deep understanding of how we design and validate biomarker assays to ensure they meet the high standards of accuracy and reliability. We’ll discuss the innovative technologies and methodologies employed to develop assays that can be seamlessly integrated into clinical practice, providing actionable insights for patient care.
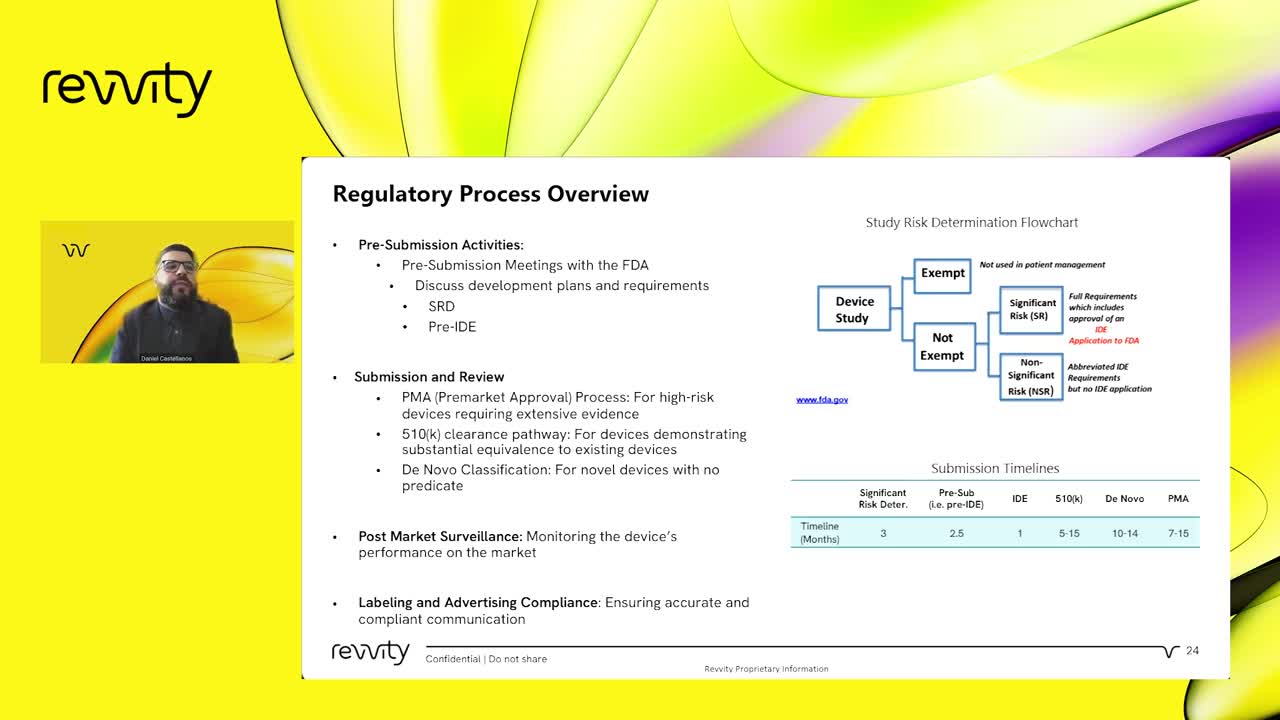
Furthermore, we’ll explore the critical steps involved in navigating the complex regulatory landscape. Our team will share insights on how to effectively manage the regulatory submission process, ensuring that your CDx solutions meet all necessary requirements for approval. From pre-submission meetings with regulatory bodies to final submission and post-market surveillance, we cover it all. Whether you are in the early stages of developing a CDx or looking to streamline your regulatory processes, this webinar will provide valuable knowledge and practical advice. Join us to learn how Revvity Omics' end-to-end solutions can help bring your CDx projects to life, ultimately driving better patient outcomes and advancing the field of precision medicine.
This testing service has not been cleared or approved by the U.S. Food and Drug Administration. Testing services may not be licensed in accordance with the laws in all countries. The availability of specific test offerings is dependent upon laboratory location. The content on this page is provided for informational purposes only, not as medical advice. It is not intended to substitute the consultation, diagnosis, and/or treatment provided by a qualified licensed physician or other medical professionals.
To view the full content please answer a few questions





























