Introduction
In recent years, small non-coding RNAs, especially microRNAs (miRNAs), have emerged as central regulators in cancer biology, playing multifaceted roles in gene expression control, epigenetic regulation, and cellular signaling networks. These ~22 nucleotide RNAs mediate post-transcriptional repression by binding to complementary sequences in mRNA targets, influencing transcript stability and translational efficiency.
Because of their direct involvement in oncogenic and tumor suppressor pathways, miRNAs have garnered increasing interest as diagnostic, prognostic, and predictive biomarkers.
The advent of next-generation sequencing (NGS) technologies has greatly enhanced our ability to characterize small RNAs in a more comprehensive and less biased manner. While early profiling technologies such as microarrays and RT-qPCR were constrained by probe design and limited throughput, NGS enables isoform-level resolution, simultaneous detection of thousands of species, and de novo discovery of novel miRNAs and isomiRs, variations of canonical miRNAs with potential functional divergence.
As cancer research becomes more precision-driven and multi-modal, small RNA sequencing is emerging as a vital tool for biomarker discovery, liquid biopsy development, and longitudinal disease monitoring. In this blog, we explore the biological foundations of miRNAs as biomarkers, the technological breakthroughs that have enabled their detection at scale, and the ongoing challenges and frontiers that define this fast-evolving field.
Why miRNAs make ideal cancer biomarkers
miRNAs control critical tumor-related processes including cell cycle progression, apoptosis, epithelial-mesenchymal transition, immune evasion, and metabolic reprogramming. For instance, the upregulation of miR-21 in various solid tumors leads to downregulation of tumor suppressors such as PTEN and PDCD4, promoting proliferation and invasion. Conversely, downregulation of the let-7 family enhances RAS expression, a classic oncogenic event.
As discussed in a previous blog, miRNAs are present and stable in the extracellular space. Their small size and association with Argonaute (AGO) proteins or encapsulation in lipid bilayers (e.g., exosomes, microvesicles) protect them from RNases, allowing their detection in biofluids long after sample collection. This makes them highly attractive candidates for non-invasive diagnostics, including blood-based liquid biopsies and urine-based monitoring for urological cancers.
Furthermore, miRNAs exhibit tissue- and disease-specific expression patterns. Large cancer transcriptome studies have revealed distinct miRNA signatures capable of differentiating cancer types, stages, and even molecular subtypes. In glioblastoma, for example, miR-10b and miR-21 are selectively upregulated and associated with poor survival, while in prostate cancer, circulating levels of miR-141 and miR-375 correlate with metastatic progression.
These features - functional relevance, remarkable stability, and tissue specificity, make miRNAs an exceptional class of biomarkers with both research and translational significance.
NGS: Enabling comprehensive small RNA profiling
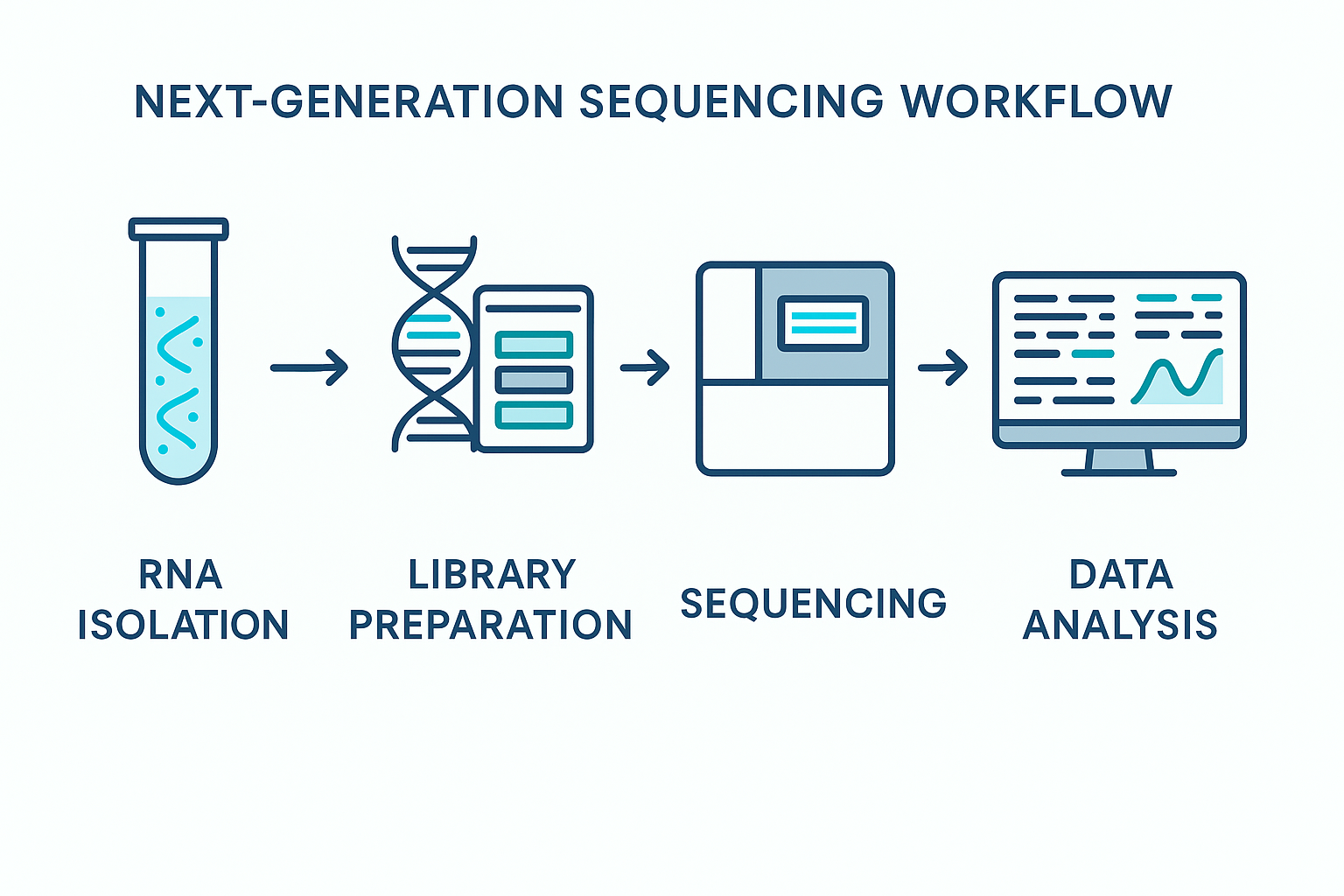
NGS-based small RNA sequencing
 NEXTFLEX Small RNA Sequencing Kit V4
overcomes many limitations of traditional profiling methods. Where RT-qPCR and microarrays require prior knowledge of miRNA sequences, NGS provides a highly comprehensive and less biased approach capable of detecting both known and novel small RNAs, including isomiRs, tRNA fragments (tRFs), piRNAs, Y RNA, and snoRNA-derived fragments, which may all contribute to cancer biology.
NEXTFLEX Small RNA Sequencing Kit V4
overcomes many limitations of traditional profiling methods. Where RT-qPCR and microarrays require prior knowledge of miRNA sequences, NGS provides a highly comprehensive and less biased approach capable of detecting both known and novel small RNAs, including isomiRs, tRNA fragments (tRFs), piRNAs, Y RNA, and snoRNA-derived fragments, which may all contribute to cancer biology.

The typical NGS small RNA-seq workflow includes adapter ligation, reverse transcription, PCR amplification, and sequencing, often on platforms such as Illumina’s MiSeq® or NovaSeq® sequencers, or increasingly, Element Biosciences and other emerging technologies.
Key technical advantages of NGS for miRNA profiling include:
- Isoform Resolution: Enables characterization of 5′ and 3′ isomiRs, which may have altered target specificity or stability.
- Dynamic Range: Quantifies miRNAs with vastly different abundances, capturing both dominant and rare transcripts.
- Throughput and Multiplexing: Permits analysis of hundreds of samples per run using unique barcodes, ideal for population-scale studies.
- Low Input Requirements: Protocols now accommodate picogram-range inputs, enabling profiling from single cells, FFPE samples, or small-volume biofluids.
The analytical flexibility provided by NGS is unmatched, offering researchers the tools to map the full spectrum of small RNA expression with high confidence and reproducibility.
Applications in oncology: Real-world impact of NGS-based miRNA profiling
NGS-based small RNA profiling has delivered critical insights across nearly every major cancer type. Their utility spans early detection, prognosis and therapeutic monitoring.
For example, in breast cancer, studies have revealed distinct miRNA profiles associated with triple-negative versus luminal subtypes, helping stratify patients and predict therapeutic responses. miR-10b, in particular, is associated with increased invasion and metastasis, while miR-205 downregulation correlates with loss of epithelial identity.
In prostate cancer, overexpression of plasma miR-21, miR-125b, miR-126, miR-141, let-7, miR-205, and miR-375 have been shown to have diagnostic relevance.
In colorectal cancer, circulating exosomal miRNAs such as miR-1246, miR-23a, and miR-1229-5p are elevated in non-responders to FOLFOX chemotherapy, offering a means to non-invasively monitor therapy resistance. Similar profiles have been reported in lung, gastric, liver, and pancreatic cancers, with clear signatures linked to hypoxia, angiogenesis, and immune evasion.
Furthermore, NGS enables the study of longitudinal dynamics in miRNA expression. By sequencing plasma samples over time, researchers can monitor how miRNA profiles change in response to surgery, immunotherapy, or relapse. This opens the door to real-time tracking of tumor evolution, a central goal of precision oncology.
A notable advantage of NGS is the ability to distinguish tumor-derived miRNAs from those of immune or stromal origin, especially when combined with single-cell RNA-seq or spatial transcriptomics. This helps resolve tumor heterogeneity and uncover microenvironmental interactions that would be lost in bulk analysis.
Technical considerations and pitfalls in small RNA-seq
Despite its utility, small RNA-seq is not without challenges. One of the most persistent issues is adapter ligation bias, caused by sequence-dependent variability in ligation efficiency. This can distort miRNA abundance estimates, especially for species with secondary structures that impede adapter annealing. Newer library prep kits have adopted degenerate bases and chemical modifications to reduce this bias, though standardization remains elusive.
Another key consideration is sequencing depth. While 5–10 million reads per sample may suffice for abundant miRNAs in cell lines, profiling low-abundance circulating miRNAs in plasma may require >20 million reads per sample to achieve reliable quantification. Without sufficient depth, biologically relevant but rare species may be missed.
Normalization and data analysis also present challenges. Traditional normalization methods like TPM or reads per million (RPM) fail to account for global shifts in expression, especially in disease states where a few highly expressed miRNAs dominate. Use of synthetic spike-ins or invariant endogenous controls is recommended, but consensus has not yet been reached.
Finally, bioinformatics analysis of small RNA-seq data is complex and often underappreciated. Accurate isomiR calling, miRNA-mRNA interaction modeling, and integration with other omics data require sophisticated pipelines and careful quality control. Public tools like miRDeep^{2}, sRNAbench, and Chimira are widely used, but customization is often necessary depending on the application.
Looking forward: Multi-omic integration and AI-driven biomarker discovery
The future of small RNA biomarker research lies in integration. As multi-omic datasets become more accessible, researchers are combining small RNA-seq with transcriptomics, epigenomics, proteomics, and even metabolomics to construct richer models of cancer biology. For example, integration of miRNA and mRNA data can uncover regulatory axes (e.g., miR-34a–MYC–CDK6) that drive tumor progression and treatment resistance.
Additionally, machine learning and AI approaches are being increasingly used to analyze high-dimensional small RNA datasets. These models can classify tumors, predict treatment response, and even anticipate immune checkpoint inhibitor efficacy based on miRNA signatures. Importantly, they also help identify subtle, combinatorial patterns across multiple biomarkers, something traditional univariate approaches often miss.
Emerging tools such as spatial small RNA-seq, single-vesicle RNA profiling, and synthetic miRNA sensors are pushing the boundaries even further, enabling researchers to localize and quantify miRNAs in their native tissue context or even in live cells.
As these innovations converge, small RNA-seq stands to become a core component of the precision oncology toolkit, enabling a new generation of biomarker discovery and translational research.
Conclusion
MicroRNAs are not just regulators of gene expression, they are deeply embedded in the architecture of cancer biology. Their biochemical resilience, functional specificity, and dynamic responsiveness to oncogenic events make them exceptional candidates for biomarker development.
NGS-based small RNA profiling provides the sensitivity, resolution, and scale required to fully explore this potential. As library preparation methods evolve, bioinformatics pipelines improve, and analytical frameworks mature, the barriers to reliable miRNA biomarker discovery are steadily falling.
With the integration of multi-omic technologies and the rise of AI-assisted analysis, the future of small RNA-based biomarkers is not just promising, it is foundational to the next wave of personalized cancer diagnostics and therapy.
References:
- Peng, Y., & Croce, C. M. (2016). The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 1, 15004.
- Witwer, K. W., & Halushka, M. K. (2016). Enhancing reproducibility and rigor in microRNA research. RNA Biol, 13(11):1103-1116.
- Frixa, T., Donzelli, S., & Blandino, G. (2015). Oncogenic microRNAs: key players in malignant transformation. Cancers, 7(4): 2466-2485.
- Weidle, U. H., et al. (2021). The emerging role of miRNAs in cancer immune escape: molecular targets and translational perspectives. Biochim Biophys Acta Rev Cancer, 1875(2), 188494. https://doi.org/10.1016/j.bbcan.2021.188494
- Rupaimoole, R., & Slack, F. J. (2022). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature Reviews Drug Discovery, 21(8), 589–608. https://doi.org/10.1038/s41573-022-00389-3