Therapeutic oligonucleotides are synthetic DNA or RNA strands designed to treat diseases. They include various types such as antisense oligonucleotides, small interfering RNAs (siRNAs), aptamers, messenger RNAs (mRNAs), and CRISPRs. These therapies are considered precise medicines because they target the genetic causes underlying diseases.
These technologies can modify RNA expression and splicing, block the production of disease-causing proteins, alter gene expression, restore the production of beneficial proteins, and help modulate immune responses. They are opening up treatment options in areas that were previously deemed undruggable using conventional medications, such as small molecules and antibodies.
How are therapeutic oligonucleotides delivered to cells?
In clinical applications, various delivery mechanisms are used for oligonucleotides, including:
- Chemical modification: Phosphorothioate backbones and 2'-O-methyl groups enhance stability, while GalNAc conjugation enables targeted liver delivery.1
- Lipid-based delivery systems: Lipid nanoparticles (LNPs), which have achieved clinical success in approved therapeutics like Onpattro® and mRNA vaccines.1
- Polymer-based carriers: PEI (polyethylenimine) complexes and, more recently, chitosan, a natural biodegradable polymer showing promise in clinical testing due to its biocompatibility and mucoadhesive properties.
- Bioconjugation: Attaching the oligonucleotide to cell-penetrating peptides or antibodies enhances delivery. Antibody-oligonucleotide conjugates (AOCs), which combine the specificity of antibodies with the therapeutic effects of oligonucleotides, offer a precise delivery mechanism for targeting specific cell types and represent an emerging approach for expanding the therapeutic reach of oligonucleotides.2
- Direct administration: Specialized routes like intrathecal injection for CNS delivery and intravitreal injection for ocular diseases bypass biological barriers for targeted therapy.
There has been a notable increase in FDA-approved therapies in the field of therapeutic oligonucleotides.3 This area is not just emerging; it is rapidly expanding, creating opportunities for collaboration with companies in this sector.
At Revvity, our preclinical services are strategically positioned to support hit identification and lead optimization, including efficiency of delivery and effects on gene expression. Our core strength lies in the use of downstream functional assays as indicators of whether therapeutic oligonucleotides have been successfully delivered into cells and how effectively they induce downstream responses.

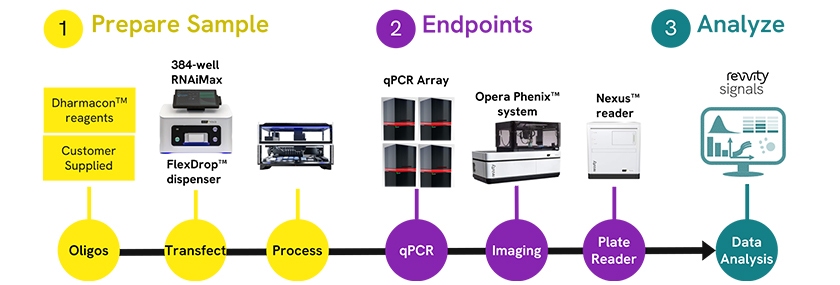
Workflow diagram depicting the three-phase process for therapeutic oligonucleotide screening: 1) Sample preparation utilizing Dharmacon™ reagents or customer materials with FlexDrop™ dispenser and 384-well RNAiMax transfection technology; 2) Endpoints Analysis via qPCR arrays, Opera Phenix™ high-content imaging, and Nexus™ plate reading; and 3) Data Analysis through Revvity Signals™ software. This integrated platform streamlines the evaluation of oligonucleotide candidates, accelerating nucleic acid-based therapeutic development.
Screen configuration
Client screens in this area typically involve large-scale primary screens of oligonucleotide libraries focused on identifying effective sequences. In these screens, clients often provide libraries containing hundreds to thousands of candidate oligonucleotides targeting a specific gene of interest. These libraries may include oligonucleotides that target different regions of the mRNA or incorporate various modifications to enhance cellular uptake, increase stability, or extend the activity duration within cells.
Hits from the primary screen are then evaluated in secondary dose response assays. These screens are often iteratively repeated as part of lead oligonucleotide optimization. While some reagents can self-deliver into cells, standard transfection reagents are used to evaluate efficacy. The primary readout for screens is typically qPCR to determine target modulation; however, other assays, such as HTRF™ and high content imaging, can also be used to assess the effects of target modulation.
Expanded qPCR capacity and cell panel choices
Screens are conducted on Revvity's high-throughput functional genomic screening platform, which benefits from the recent addition of a new 384-well qPCR machine array consisting of 10 instruments, expanding capacity to process thousands of samples per week and markedly shortening project timelines. Clients can select cell lines from Revvity's extensive cell line collections. Large panels can also be screened to determine the breadth of activity and support patient stratification.
Conclusion
Revvity’s preclinical services team is exceptionally well-positioned to support therapeutic oligonucleotide screening programs. Our established expertise in functional genomic screening aligns perfectly with the needs of this rapidly growing field. With our expanded qPCR capabilities, diverse cell model systems, and proven track record of successful collaborations, we offer comprehensive solutions for clients at any stage of oligonucleotide therapeutic development.
We have successfully implemented our services across various applications, from primary screening of large oligonucleotide libraries to evaluating novel delivery technologies in different tissue types. The flexibility of our platform enables customization to meet specific client needs, whether it involves optimizing lead candidates, assessing delivery efficiency, or characterizing mechanisms of action.
As the therapeutic oligonucleotide market continues to expand rapidly, Revvity is equipped with the experience, technology, and scientific expertise to accelerate development programs and help bring these innovative medicines to individuals.
Accelerate your oligonucleotide therapeutic program. Contact our scientific team today to discuss how our high-throughput screening platform and expanded qPCR capacity can support your development needs.
References:
- Egli M, Manoharan M Wilson K, Garima, Dhanawat M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Research 2023, April 11; 51(6):2529-2573. doi:10.1093/nar/gkad067
- Bian X, Zhou L, Luo Z, Liu G, Hang Z, Li H, Li F, Wen Y. Emerging Delivery Systems for Enabling Precision Nucleic Acid Therapeutics. ACS Nano. 2025 Feb 4;19(4):4039-4083. doi: 10.1021/acsnano.4c11858.
- Mullard, A. 2024 FDA Approvals. Nature. 2025, Jan. 02. doi: https://doi.org/10.1038/d41573-025-00001-5


































