CD8 T cells naturally have the ability to recognize and eliminate target cells through antigen-dependent mechanisms. This T cell-mediated killing occurs when T cells recognize infected or malignant cells via antigens on their cell surface and induce killing of these cells via several mechanisms, including the release of cytotoxic granules and cell death-inducing cytokines.
Harnessing the natural ability of immune cells to recognize and eliminate malignant cancer cells is the foundation of immunotherapy research. However, accurately capturing and quantifying T-cell activity has historically been challenging. Traditional cytotoxicity methods often rely on indirect measurements of cell death, which limits real-time analysis. To overcome these limitations, researchers need robust platforms and adaptable tools to assess the activity of cytotoxic T cells against cancer cells of interest.
As research evolves, imaging-based cytotoxicity assays have emerged as a powerful method for exploring the interactions between immune cells and cancer cells in real time. Unlike traditional methods, imaging-based cytotoxicity assays enable direct visualization of cellular viability parameters in real time. This allows researchers to assess T-cell activation and monitor the capacity of immune cells to target and eliminate tumor cells.
Imaging cytotoxicity assays to evaluate a DGK inhibitor impact on T cell function
In a notable study published in 2023 by researchers from Harvard, Dana Farber Institute, Massachusetts General Hospital and Bristol Myers Squibb, imaging cytotoxicity assays utilizing the Celigo Imaging Cytometer were employed to investigate the impact of a diacylglycerol kinase inhibitor (DGKi) on T cell function.
Previous studies have shown that DGK negatively regulates T cell activity, highlighting the potential of DGK inhibition to enhance tumor-specific T cell responses. However, existing DGK inhibitors have limited therapeutic efficacy as they target only one of the two isoforms, have low affinity, and exhibit off-target effects.
To determine if this is an effective anti-tumor therapy, the researchers tested a novel DGK inhibitor (DGKi) in multiple experiments using CD8⁺ T cells specific for an epitope from the melanoma self-antigen tyrosinase-related protein 1 (Tyrp1), with varying TCR affinities. Among the approaches used, imaging cytometry enabled direct measurement of T cell-mediated killing of cancer cells under different conditions, demonstrating the utility of this technology for real-time functional analysis of immune effector mechanisms. Overall, the data indicate that DGK inhibition enhances T cell activity, particularly in contexts of low TCR affinity or avidity—conditions commonly seen in tumors with low mutational burden.
DGKi enhances T cell-mediated killing
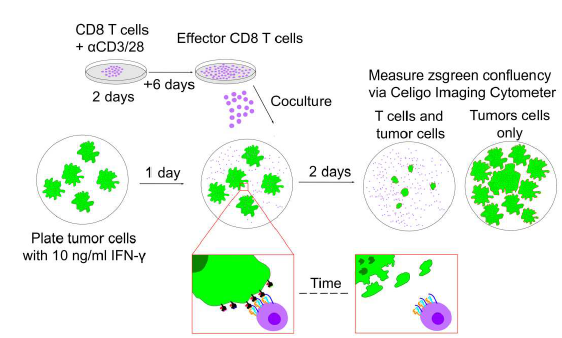
To directly assess T cell mediated killing of cancer cells, the researchers developed an in vitro T cell cytotoxicity assay using B16 melanoma and 6694c2 pancreatic cancer cell lines engineered to express zsGreen (Figure 1). The 6694c2 cells were also transduced to express low amounts of Tyrp1, and this new cell line was dubbed C2VTrp1. These cell lines were co-cultured with either TRP1high or TRP1low effector cells, which have different affinities for the Tyrp1 antigen, in the presence or absence of DGKi. Killing was directly assessed with the Celigo Image Cytometer by quantifying the change of Zsgreen confluency over time and comparing the results to tumor only control wells.

Figure 1: Celigo cytotoxicity assay schematic. Effector T cells are cultured for 8 days before co-culture with tumor cells pre-treated with IFN-y. Confluency is assessed and compared to tumor-only control wells. Figure adapted from Kureshi et al. licensed under CC BY 4.0.
Results showed that DGKi treatment significantly increased the killing capacity of both effector cell populations against C2VTrp1 cells and enhanced the ability of TRP1high effector cells to kill 6694c2 parental cells.
The authors subsequently conducted several additional experiments to assess the impact of DGKi on T cell functions using the Celigo, including elucidation of the mechanism and effect of exposure timing (Figure 2). These experiments used either the Zsgreen readout or a Caspase-3 reporter dye to measure apoptosis. The signal from this dye can also be assessed using the Celigo instrument.
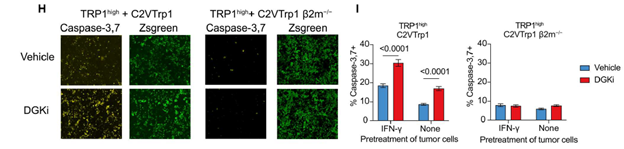
Figure 2: Images of Caspase-3 and Zsgreen reporters after 48 hours of co-culture of effector and tumor cells. For these experiments, the authors generated MHC-1 deficient C2VTrp1 cells by CRISPR-Cas9 deletion of the B2m gene. They also pre-treated the tumor cells with IFN-y to induce MHC-1 expression, which enhanced Caspase-3 reporter signal in the C2VTrp1 cells with the intact B2m gene, while the cells with the deleted B2m gene showed no effect. The Caspase-3 signal in the C2VTrp1 cells was further enhanced in the presence of DGKi, indicating that while DGKi decreases the threshold needed for T cell activation, MHC-1 is still required. Figure adapted from Kureshi et al. licensed under CC BY 4.0.
Conclusion
Imaging cytometry is a highly useful tool for in vitro T cell immunology studies. The experiments in this paper by Kureshi et al. highlight the utility of the Celigo imaging cytometer for in vitro killing assays. The authors were able to evaluate multiple parameters, including the effect of varying TCR affinity to the target antigen, the mechanism of TCR signaling and the role of MHC-1, the mechanism of T cell cytotoxicity, and the role of DGKi exposure timing.
Taken together, these experiments demonstrate the high utility of the Celigo in immunotherapy studies. To learn more about how the Celigo imaging cytometer can enhance your T cell functional assays and accelerate your immunotherapy research, visit our product page.
Reference:
- Kureshi R, Bello E, Kureshi CTS, Walsh MJ, Lippert V, Hoffman MT, Dougan M, Longmire T, Wichroski M, Dougan SK. DGKα/ζ inhibition lowers the TCR affinity threshold and potentiates antitumor immunity. Sci Adv. 2023 Nov 24;9(47):eadk1853. doi: 10.1126/sciadv.adk1853. Epub 2023 Nov 24. PMID: 38000024; PMCID: PMC10672170.
For research use only. Not for use in diagnostic procedures.