The idea that fragments of DNA circulate outside cells dates to 1948, when Mandel and Métais reported "acides nucléiques" in human serum1. Half a century later, Lo et al. demonstrated that placenta-derived DNA is present in maternal plasma, establishing that tissue-specific DNA can be found in this pool2. Those discoveries reframed peripheral blood as a molecular “mirror” of internal organs. By the early 2010s, this vision was made popular under the term “liquid biopsy”, to encompass any test that interrogates disease through circulating analytes such as cell free DNA (cfDNA), circulating tumour cells, or extracellular vesicles3. cfDNA has been found in almost every body fluid that has been systematically examined.
In healthy individuals, plasma cfDNA derives primarily from apoptosis of normal cells of the hematopoietic lineage, with minimal contributions from other tissues. In specific physiological conditions or disease processes, a proportion of cfDNA may be derived from different tissues4.
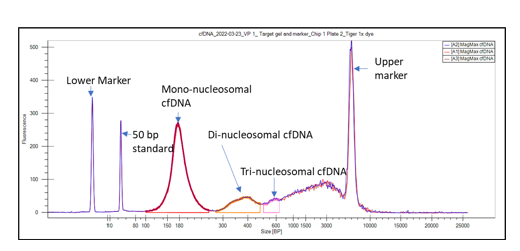
The size distribution of cfDNA fragments, peaking at ~167 bp, the length of DNA wrapped around a mononucleosome plus linker, is consistent with this apoptotic origin. High-resolution sizing (Figure 1) further reveals regularly spaced peaks at ~334 bp, ~501 bp, etc., corresponding to di- and tri-nucleosomes5. Biophysical studies and immunocapture assays have shown that most of these molecules circulate still wrapped around nucleosomes6.

Figure 1. Typical cfDNA sizing profile using the LabChip® GX Touch™ Nucleic Acid Analyzer
 LabChip cfDNA Assay
LabChip cfDNA Assay
The fragments that dominate plasma-derived cfDNA are double stranded molecules with single-stranded protruding ends of variable length (6-10 nucleotides), known as jagged ends. All fragments present 5'-phosphate, and 3'-hydroxyl end typical of nuclease cleavage. The sequence of the fragment termini is not random: certain 4 bases motifs (e.g., CCGA, CCGG) are enriched, reflecting the sequence preferences of plasma nucleases such as DNase1L37.
Beyond canonical mononucleosomal DNA, other circulating DNA molecules can be found in plasma:
- Long/open chromatin fragments: rare fragments >1 kb can be recovered inside extracellular vesicles or apoptotic bodies; they represent loosely packed chromatin loops or even extrachromosomal circular DNA as shown by topology-resolved studies. They are originated by necrosis, apoptotic body release or active vesicular export8.
- Ultrashort cfDNA (< 100 nt): A distinct population of ~50–70 nt molecules that is largely single-stranded. It was only recently detected thanks to the use of specialized single-stranded library preparations. They are enriched in mitochondrial, microbial, and tumour DNA. They can result from active secretion or neutrophil extracellular trap formation9.
- Mitochondrial cfDNA (16.6 kb): Mitochondria contribute both full-length circular genomes encapsulated in vesicles and shorter double-stranded or single-stranded pieces. The size distribution is often broader and more heterogeneous than nuclear cfDNA, and fragmentation hotspots track mitochondrial nucleoids rather than nucleosomes. Aberrant mitochondrial cfDNA fragmentation patterns have been linked to inflammatory states and cancer10.
Dynamic cfDNA regulation in health and disease
| Context | Change vs. healthy baseline | Dominant mechanism |
|---|---|---|
| Pregnancy | ↑ 5- to 10-fold | Placental apoptosis |
| Early-stage cancer | Slight ↑ | Tumour cell turnover |
| Advanced cancer | ↑↑ (often > 10 ng/mL) | Apoptosis + necrosis |
| Severe trauma | ↑↑ correlated with Injury Severity Score12 | Tissue injury |
| Strenuous exercise | Transient ↑ peaking within 30 min13 | NETosis* & muscle stress |
| Acute graft rejection | Donor-derived fraction > 1%14 | Graft injury |
Table 1. Changes in cfDNA concentration in different conditions
*Neutrophil extracellular trap formation
The fact that total cfDNA rises in predictable patterns gives researchers a uniquely non-invasive way to peer inside tissues that would otherwise be inaccessible without surgery or biopsy. For example, a modest but reproducible rise in tumour-derived cfDNA can precede radiographic evidence of relapse by weeks15, while a spike in donor-specific cfDNA after organ transplantation signals rejection before serum creatinine or biopsy changes are evident16. Likewise, the appearance of placenta-derived fragments in maternal plasma underpins modern non-invasive prenatal testing, which now is used in millions of pregnancies every year.
Despite these advances the detection of a tissue-specific signal in a large background of "housekeeping" cfDNA released by normal cell turnover is still the main challenge. Even in advanced cancer, tumor derived cfDNA may represent < 1% of the total cfDNA pool; in early-stage disease or minimal residual disease the fraction can fall below 0.01%.
Analytical techniques used to study cfDNA
Table 2 shows a list of cfDNA‐focused assays that have demonstrated limits of detection (LoD) ≤ 0.1 % variant-allele frequency (VAF).
| Technique | Principle | Breadth | Published LoD |
|---|---|---|---|
| Droplet digital PCR (ddPCR) | Millions of water-in-oil droplets → single-molecule PCR → Poisson counting of mutant vs. wild-type droplets | 1–4 loci per reaction | 0.05% typical. Down to 0.01 % VAF for well-validated hot spots.17 |
| BEAMing | Emulsion PCR on magnetic beads followed by flow-cytometric counting | 1–2 hot-spots | 0.02% VAF18 |
| Safe-SeqS | UMI-tagged amplicons; consensus of duplicates | 10–50 kb | 0.1% VAF19 |
| Amplicon Panels | Two-step PCR with UMI barcodes; sequencing on Illumina/Ion platforms | 150–300 kb | 0.1% VAF20 |
| UMI-based hybrid-capture (e.g., TruSight Oncology 500 ctDNA) | UMIs + UDIs suppress PCR and index hopping | 0.5–2 Mb | 0.2 -0.5% VAF21 |
| iDES-CAPP-Seq | Personalised hybrid capture + in-silico & UMI error modelling | 200–500 kb | 0.004–0.01% VAF in NSCLC plasma22 |
| Whole Genome Sequencing (WGS) | Complementary strands carry matched UMIs; duplex consensus | whole genome | ≤ 0.01% VAF across the genome23 |
| CRISPR/Cas-based HiCASE assay | Cas13a collateral cleavage with mutation-specific probes | 1 hot-spot | 0.02% VAF in cfDNA standars24 |
Table 2. List of currently available approaches to study cfDNA
The technique of choice depends on the goals of the study. If the researcher is interrogating ≤10 loci and needs a fast turnaround time, then ddPCR/BEAMing are the best approach. If the goal is to investigate a few hundred of loci then targeted approaches such us Safe-SeqS, Amplicon panels or UMI-based hybrid-capture should be favoured. However, if the goal is to obtain a comprehensive mutation and CNV profiling then WGS is the obvious option.
When cfDNA is prepared with NEXTFLEX™ Cell-free DNA-Seq Library prep 2.0
 NEXTFLEX Cell Free DNA-Seq Library Prep Kit 2.0
and the NEXTFLEX UDI-UMI Barcodes
NEXTFLEX Cell Free DNA-Seq Library Prep Kit 2.0
and the NEXTFLEX UDI-UMI Barcodes
 NEXTFLEX UDI-UMI Barcodes (1-8)
, every ~160 bp plasma molecule is individually barcoded, with the forward and reverse strands sharing the same UMI, while simultaneously assigning an orthogonal i5/i7 UDI pair that is unique to each sample.
NEXTFLEX UDI-UMI Barcodes (1-8)
, every ~160 bp plasma molecule is individually barcoded, with the forward and reverse strands sharing the same UMI, while simultaneously assigning an orthogonal i5/i7 UDI pair that is unique to each sample.
After sequencing, reads bearing the same genomic coordinates and UMI are collapsed into a molecule-level consensus sequence that suppresses polymerase and sequencing errors typically by two orders of magnitude, allowing confident detection of tumour-, placental- or graft-derived variants present at just a few parts per thousand in an overwhelming wild-type background.
Practical experience shows that ~25,000 × raw coverage on a targeted panel, or ~120 × raw for whole-genome libraries, returns ~4,000 × UMI-deduplicated depth, sufficient to call single-nucleotide variants down to ~0.1% VAF for minimal-residual-disease or transplant monitoring. Low-pass WGS at 0.5–1 × deduplicated depth still supports copy-number and aneuploidy analysis, while applications that aim for ~0.05% VAF (e.g., early-relapse surveillance) benefit from pushing raw depth toward 200 ×.
Concluding remarks
From their discovery in 1948 to current roles in oncology, transplantation and prenatal medicine, cfDNA fragments exemplify how basic biology can translate into minimally invasive diagnostics. Yet their full potential emerges only when technical noise is driven far below biological signal. Whole-genome sequencing that integrates UMIs for molecular error suppression and UDIs for sample purity now provides that precision, opening avenues for earlier detection, real-time therapy monitoring and comprehensive multi-omics from a single blood draw.
References:
- Mandel P, Métais P. (1948). Les acides nucléiques du plasma sanguin chez l’homme. C R Seances Soc Biol Fil. 142(3-4):241-243.
- Lo Y M D et al. (1997). Presence of fetal DNA in maternal plasma and serum. Lancet. 350:485-487
- Alix-Panabières C, Pantel K. (2021). Liquid biopsy: from discovery to clinical application. Clin Chem. 11(4):858-873.
- Snyder, M.W., et al. (2016). Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell.164(1-2):57-68.
- Sanchez, C., et al. (2021). Circulating nuclear DNA structural features, origins, and complete size profile revealed by fragmentomics. JCI Insight. 6(7):e144561.
- Tejerina-Miranda, S., et al. (2025). Determining and characterizing circulating nucleosomes in advanced cancer with electrochemical biosensors assisted by magnetic supports and proteomic technologies. Biosens Bioelectron. 286:117582.
- Ding, S.C., et al. (2022). Jagged ends on multinucleosomal cell-free DNA serve as a biomarker for nuclease activity and systemic lupus erythematosus. Clin Chem. 68(7):917-926.
- Pisareva, E., et al. (2023). Comparison of the structures and topologies of plasma-extracted circulating nuclear and mitochondrial cell-free DNA. Front Genet.14:1104732.
- Cheng, J., et al. (2022). Plasma contains ultrashort single-stranded DNA in addition to nucleosomal cell-free DNA. iScience. 25(7):104554.
- Liu, Y., et al. (2024). Aberrant fragmentomic features of circulating cell-free mitochondrial DNA as novel biomarkers for multi-cancer detection. EMBO Mol Med.16(12):e00163.
- Mattox, A.K., et al. (2023). The Origin of Highly Elevated Cell-Free DNA in Healthy Individuals and Patients with Pancreatic, Colorectal, Lung, or Ovarian Cancer. Cancer Discov. 13(10):2166-2179.
- Trulson, I., et al. (2023). Cell-Free DNA in Plasma and Serum Indicates Disease Severity and Prognosis in Blunt Trauma Patients. Diagnostics (Basel). 17;13(6):1150.
- Rodrigues, k.B., et al. (2024). Exercise intensity and training alter the innate immune cell type and chromosomal origins of circulating cell-free DNA in humans, Proc. Natl. Acad. Sci. U.S.A. 122 (3) e2406954122.
- Aubert, O., et al. (2024) Cell-free DNA for the detection of kidney allograft rejection. Nat Med 30, 2320–2327.
- Abdelrahim, M., et al. (2025). Feasibility of Personalised and Tumour-Informed Circulating Tumour DNA Monitoring. JCO Precis Oncol. 9:e2400934
- Bromberg, J.S. et al. (2024) Elevation of Donor-derived Cell-free DNA Before Biopsy-proven Rejection in Kidney Transplant. Transplantation. 108(9):1994-2004.
- Turabi, K., et al. (2024). Decoding the Dynamics of Circulating Tumor DNA in Liquid Biopsies. Cancers (Basel). 16:2432.
- Kim, H., Park, K.U. (2023) Clinical Circulating Tumor DNA Testing for Precision Oncology. Cancer Res Treat. 55:351-366.
- Elazezy, M., Joosse, S.A. (2019). Techniques of Using Circulating Tumor DNA as a Liquid Biopsy Component in Cancer Management. Comput Struct Biotechnol J. 16:370-378.
- AmpliSeq HD Technology | Thermo Fisher Scientific - ES
- TruSight Oncology 500 ctDNA v2 | Enable CGP from ctDNA in blood plasma
- Newman, A. M., et al. (2016). Integrated Digital Error Suppression for Improved ctDNA Detection (iDES-CAPP-Seq). Nat Biotechnol. 34:547-555.
- Bae, J. H., et al. (2023). Single Duplex DNA Sequencing with CODEC Detects Mutations with High Sensitivity. Nat Genet. 55:871-879.
- Wang, L., et al. (2024). CRISPR/Cas13a-Based Supersensitive Circulating Tumor DNA Assay (HiCASE). Commun Biol.7:657
For research use only. Not for use in diagnostic procedures.