GxP Software Kit for LabChip GX and GXII Touch
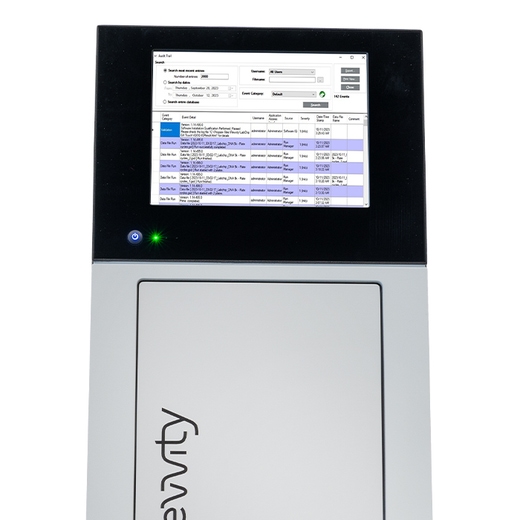


Product information
Overview
The LabChip GxP Software Kit delivers robust technical controls to ensure full compatibility with 21 CFR Part 11 requirements for electronic records and signatures. Designed for regulated environments, it safeguards assay data, analysis settings, event logs, and backup files from unauthorized editing or tampering. All data is securely stored in designated folders on the LabChip GX Touch computer, LabChip Reviewer computer, or a network server. The solution leverages two core components: the Central Data Repository (CDR) and the CFR database. The CDR provides a secure location for assay and data files, while the CFR database manages user accounts, audit trails, and data folder information. To maintain compliance and data integrity, the kit includes Microsoft® SQL Server® Express for creating and managing the CFR database, ensuring validated workflows and traceable audit history.
Specifications
| Brand |
LabChip GX
LabChip GXII
|
|---|---|
| Unit Size |
1 unit
|
Resources
Are you looking for resources, click on the resource type to explore further.
Learn how the LabChip systems automate DNA, RNA, & protein analysis with GxP Software for compliance, access control, & data...


How can we help you?
We are here to answer your questions.