HTRF Human Total cGAS Detection Kit, 500 Assay Points







| Feature | Specification |
|---|---|
| Application | 細胞シグナル伝達 |
| Sample Volume | 16 µL |









Product information
Overview
Following pathogen infection and the binding of dsDNA to the cytoplasmic sensor cGAS, STING protein is phosphorylated by TBK1, enabling its binding to IRF3 which leads to IFNs type 1 production. The STING pathway is then switched off by STING degradation, involving autophagy.
In immuno-oncology, activating the STING pathway has shown promising anti-tumor effects in pre-clinical models and thus represents a therapeutic strategy to treat human cancer.
How it works
Total cGAS assay principle
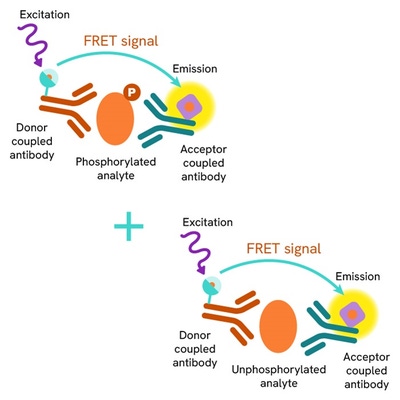
The Total cGAS assay quantifies the expression level of cGAS in a cell lysate. Unlike Western Blot, the assay is entirely plate-based, and does not require gels, electrophoresis, or transfer. The Total cGAS assay uses two labeled antibodies, one coupled to a donor fluorophore, the other to an acceptor. Both antibodies are highly specific for a distinct epitope on the protein. In presence of cGAS in a cell extract, the addition of these conjugates brings the donor fluorophore into close proximity with the acceptor, and thereby generates a FRET signal. Its intensity is directly proportional to the concentration of the protein present in the sample, and provides a means of assessing the protein’s expression under a no-wash assay format.

Total cGAS two-plate assay protocol
The two-plate protocol involves culturing cells in a 96-well plate before lysis, then transferring lysates into a low volume detection plate (either HTRF 384-lv or 96-lv plate) before the addition of HTRF Total cGASdetection reagents. This protocol enables the cells' viability and confluence to be monitored.

Total-ATG16L1 one-plate assay protocol
Detection of Total cGAS with HTRF reagents can be performed in a single plate used for culturing, stimulation, and lysis. No washing steps are required. This HTS designed protocol enables miniaturization while maintaining robust HTRF quality.

Assay validation
Validation of Total cGAS detection in siRNA-treated THP-1 cells
THP-1 cells were plated at 100,000 cells per well under 90µl in a 96-well plates in complete culture medium. For Lipofectamine treated samples,10µl of Lipofectamine 1%f in culture medium were added. For siRNA treated cells, a 10µl mixture of Lipofectamine® RNAiMax/siRNA for cGas was added. Cells were then incubated for 48 h and 72 h at 37°C, 5% CO2.
After incubation, cells were lysed with 33.3µL of supplemented lysis buffer #1 at 4X for 30 minutes at RT under gentle shaking, and 16µL of lysate were transferred into a low volume white microplate before adding 2µL of the HTRF d2 detection reagent and2 µL HTRF Eu-K detection reagent. The HTRF signal was recorded after an ON incubation.

Validation of Total cGAS detection in siRNA-treated HeLa cells
HELA cells were plated at 50,000 cells per well under 90µl in a 96-well plates in complete culture medium. For Lipofectamine treated samples,10µl of Lipofectamine 1%f in culture medium were added. For siRNA treated cells, a 10µl mixture of Lipofectamine® RNAiMax/siRNA for cGas was added. Cells were then incubated for 24 h and 48 h at 37°C, 5% CO2.
After incubation, cells were lysed with 90µL of supplemented lysis buffer #1 at 1X for 30 minutes at RT under gentle shaking, and 16µL of lysate were transferred into a low volume white microplate before adding 2µL of the HTRF d2 detection reagent and 2µL HTRF Eu-K detection reagent. The HTRF signal was recorded after an ON incubation.

Specifications
| Application |
Cell Signaling
|
|---|---|
| Brand |
HTRF
|
| Detection Modality |
HTRF
|
| Lysis Buffer Compatibility |
Lysis Buffer 1
|
| Molecular Modification |
Total
|
| Product Group |
Kit
|
| Sample Volume |
16 µL
|
| Shipping Conditions |
Shipped in Dry Ice
|
| Target Class |
Phosphoproteins
|
| Target Species |
Human
|
| Technology |
TR-FRET
|
| Unit Size |
500 assay points
|
Video gallery
Resources
Are you looking for resources, click on the resource type to explore further.
Discover the versatility and precision of Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our HTRF portfolio offers a...


How can we help you?
We are here to answer your questions.