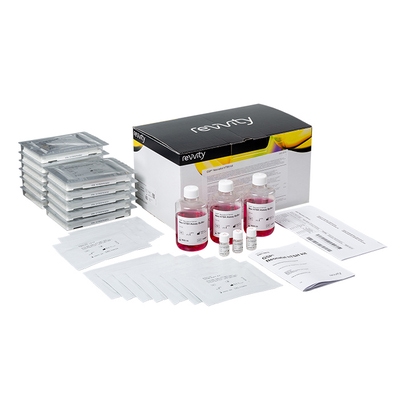
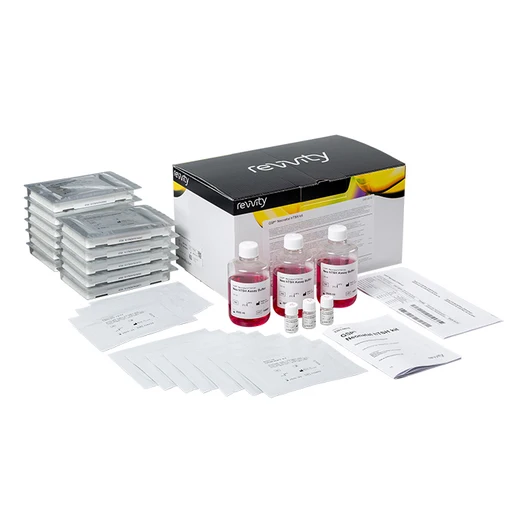
GSP Neonatal hTSH kit
| Feature | Specification |
|---|---|
| Application | 新生児スクリーニング |



Product information
Overview
Congenital hypothyroidism (CH) results from a failure of the thyroid glands to produce thyroid hormones in adequate amounts. The condition can easily be treated with daily doses of thyroid hormones but clinical diagnosis is difficult to establish and the disease may continue unrecognized for a long time causing irreversible brain damage. However, increased thyroid stimulating hormone (hTSH) and decreased thyroxine are clear signs of CH, which have led to the establishment of large scale screening programs.
GSP Neonatal hTSH assay:
- The incubation time is only 3.5 h
- Sensitive, robust DELFIA chemistry for confidence in results
- All reagents are ready to use
- The kit contains reagents and plates for 1152 tests (12 plates)
The assay is based on a direct sandwich technique where two monoclonal antibodies recognize separate antigenic determinants on the hTSH molecule. The fluorescence signal is proportional to the analyte concentration in the sample.
Features/Benefits:
- The incubation time is only 3.5 h/li>
- Sensitive, robust DELFIA chemistry for confidence in results/li>
- All reagents are ready to use/li>
- The kit contains reagents and plates for 1152 tests (12 plates)
Specifications
| Application |
Newborn Screening
|
|---|---|
| Brand |
GSP®
|
| Detection Modality |
Time-Resolved Fluorescence (TRF)
|
| Disorders |
Congenital Hypothyroidism
|
| Instrument Compatibility |
GSP
|
| Quantity |
1152 tests
|
| Sample Type |
Dried blood spots
|
| Technology |
DELFIA
|
| Unit Size |
1 kit
|
Resources
Are you looking for resources, click on the resource type to explore further.


How can we help you?
We are here to answer your questions.