Eonis Q96 (IVD)
The Eonis™ Q 96-well variant instrument has been designed to offer a simple and unique workflow, making training and implementation of qPCR easy to deliver.
With a simplified workflow, Eonis Q achieves sub-3-hour turnaround times without the need for a clean room.















Eonis Q qPCR workflow and Eonis SCID-SMA kit delivers
An efficient workflow that minimizes dependence on additional resources
Designed to function without complex laboratory instrumentation.
Small physical footprint and easy to maintain
Fewer instruments help reduce service and maintenance costs.
One easy-to-use platform helping to maintain and expand your screening program
Making it easier to train the platform, system, workflow and any new analytes.
Newborn screening focused software
A unified workstation and analysis software solution that makes sample processing simple and efficient.
Eonis EASI software - designed for newborn screening
Quick time to report
Fast reporting: no manual data handling that requires extra time.
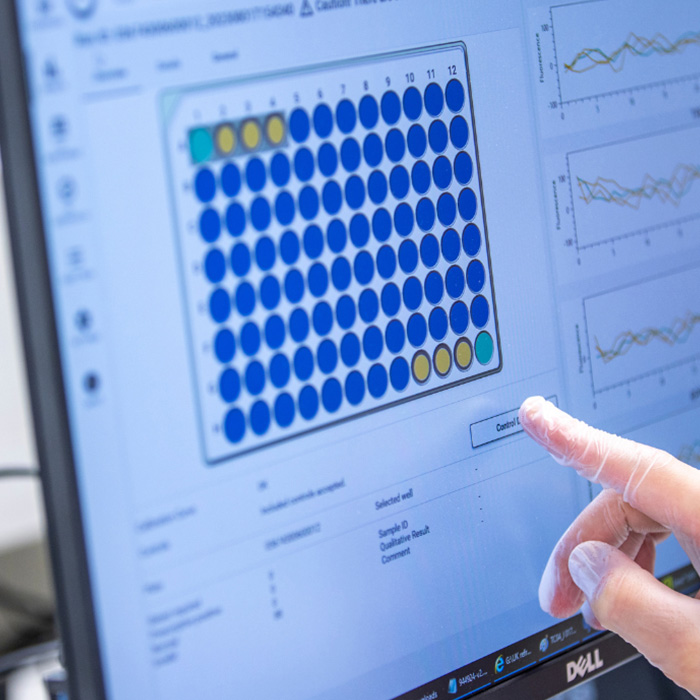
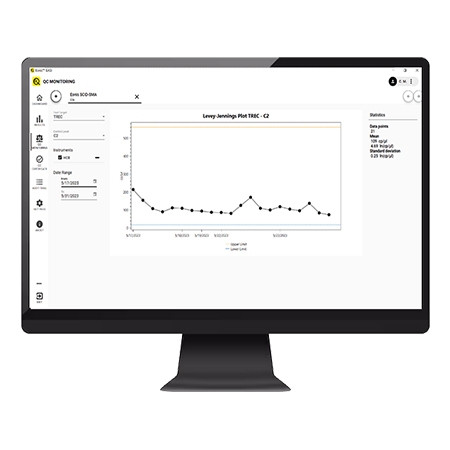
Quality control monitoring
Built in QC monitoring ensures you can track your QC levels over time: no need to keep any separate files for tracking.
Uniquely customized for newborn screening
Easy to use without any previous experience of qPCR software. The same software is used for managing the PCR run and analyzing results.
Seamless integration
Connects to LIMS system easily with no extra tools needed to run analysis.
Troubleshooting packages
With just one click, you can easily share files with our customer support if you need assistance.
Supporting you with your transformation
Revvity delivers trusted newborn screening instruments and reagents backed by 75+ years of expertise.
We provide expert-led diagnostics training to support your success with Revvity NBS solutions.
Revvity's certified service engineers deliver trusted, high-quality support to ensure peak diagnostic performance.
Specialist and support teams deliver regional help, giving you the right expertise when and where needed.
The Eonis Q system workflow
Key features
Simple and efficient, supporting a low-resource MDx workflow.
Small, compact footprint and easy maintenance translate to fewer instruments needed and lower service costs.
One unified platform that's intuitive to use, simple to maintain and scale, and makes training employees on the system much more straightforward.
NBS-focused software that combines workstation and analysis capabilities for streamlined sample processing.
Eonis Q dry chemistry qPCR workflow
Punch

DNA elution

Transfer DNA

Run & analyse PCR protocol


Eonis SCID-SMA kit
A PCR kit for qualitative detection of Spinal Muscular Atrophy (SMA) and quantitative determination of Severe Combined Immunodeficiency (SCID) and X-linked Agammaglobulinemia (XLA) using newborn dried blood spot samples.
Applications
SMA causes severe muscle weakness & respiratory failure. Early detection through newborn screening enables life-saving treatments before symptoms appear, dramatically improving outcomes.

EASI software
EASI Software is a user-friendly dedicated newborn screening solution, streamlines processes with its easy-to-use interface, eliminating the need for prior qPCR software experience and additional tools.
EASI software seamlessly integrates with hospitals' LIMS, conducts QC monitoring for tracking over time, and offers trouble-shooting packages for effortless customer support collaboration.
Uniquely customized for newborn screening laboratories
- Easy to use without any previous experience of qPCR software
- Both the PCR workflow and result analysis are controlled through one unified software
Seamless integration
- Connects to LIMS system easily with no extra tools needed to run the analysis
Time to report
- Fast reporting: no manual data handling, that requires extra time
Troubleshooting packages
- With just one click, you can easily share files with our customer support if you need assistance
Quality control monitoring
- Built in QC monitoring ensures you can track your QC level over time: no need to keep any separate files for tracking
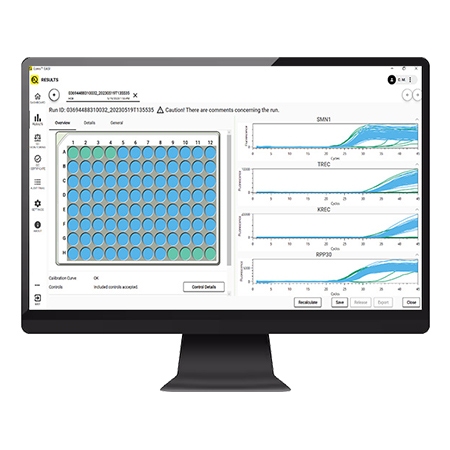
The Eonis Q system and workflow has been designed to make screening for SCID and SMA easier by optimizing time, space, cost and training.
FAQs
-
Does the assay detect all variants of SMA, SCID and XLA?
The assay detects only homozygous SMN1 exon 7 deletions. The system does not detect SMA carriers or SMA that that is not due to the homozygous deletion of SMN1 exon 7.
The assay does not detect SCID-like Syndromes, such as DiGeorge Syndrome, or Omenn Syndrome, and less acute SCID syndromes such as leaky-SCID or variant SCID.
The system does not detect atypical XLA and XLA carriers.
-
I would like to see only SMA results instead of the whole multiplex SMA-SCID-XLA panel. Can the results be filtered?
Yes, any subset of analytes can be detected with the IVD kit. The subset of analytes is selected directly from the EASI software, and the customer cannot see or restore any results from targets that have not been selected.
-
What are the space requirements? Can Eonis Q96 instrument be placed in the same room with punching and elution?
Eonis Q system workflow is designed for laboratories with limited laboratory space. Ensure that the laboratory has at least two separate laboratory areas for the workflow. Place punching, elution, and PCR plate pipetting to one area (pre-PCR) and Eonis Q96 on the other area (PCR area) to minimize the contamination risk.
-
Can other qPCR instruments than Eonis Q96 be used with the Eonis SCID-SMA dry qPCR kit?
The qPCR reagents are pre-dried in full-skirted 96-well PCR plates. These plates will fit into qPCR instruments that take in full-skirted PCR plates, but other instruments than Eonis Q96 cannot be used for TREC or KREC quantification. The lot-specific factory-generated calibration curves are created with Eonis Q96 instrument, thus quantitative results might be affected if other qPCR instruments are used.
-
Does Eonis Q PCR cycler need regular calibration or maintenance by the customer?
The overlapping spectrums of different fluorescent dyes are handled by assay specific color compensation. Thus, regular calibrations of the instrument are not needed. Preventive maintenance with optical tests is done yearly by a Revvity service engineer.
-
How are TREC, KREC and SMN1 cut-offs defined?
Customers will need to determine the lower limits of TREC and KREC cp/µl results. Usually, these limits are derived from their own validation study and based on normal population percentiles. SMN1 and RPP30 have pre-defined Ct limits which are described in the kit insert.
-
Can additional controls be used with Eonis SCID-SMA dry qPCR kit?
The kit has been designed to contain all necessary controls, but the software allows you to analyze other controls as well. However, performance claims cannot be made about unknown control types. Any additional controls should be in DBS format and handled similarly to other samples throughout the workflow.
Product information
Overview
Eonis Q96 is a real-time PCR instrument designed for easy integration into automated workflows.
The Eonis Q instrument uses dried blood spot samples (DBS samples) punched from DBS cards. The DNA from the punches are extracted with a simple extraction protocol. No wash steps are required, only a 20 minute-incubation in a simple incubator.
The Eonis Q PCR cycler includes dedicated, LIMS-compatible analysis software for result analysis and reporting.
Additional product information
Ordering information
Instruments
| Product name | Product number |
|---|---|
| Eonis Q96 instrument | 2044-0010 |
| Eonis EASI software | 2044-3010 |
| Eonis Q96 Dx EASI software licence | 2044-3020 |
| Eonis Q PC, monitor and barcode reader | 2044-8010 |
| 96-well optical test plate (for installation and PM only) |
X04-10-310P-105-14 |
| TriNEST microplate incubator shaker | 1296-0050 |
Reagents
| Product name | Description | Product number |
|---|---|---|
| Eonis SCID-SMA kit | 4x PCR plates; 96 reactions/plate, 88 samples/plate 1x bottle Elution Solution 2x Kit controls 1x Zero control |
3241-0020 |
Consumables
| Product name | Description | Product number |
|---|---|---|
| DNA elution plates | 20 plates | 4183-0010 |
| Adhesive foil seal | 100 plates | 4156-0010 |
| Optical PCR seal | 100 plates | 4197-0010 |
Specifications
| Dimensions | 310.0 mm (W) x 345.0 mm (D) x 613.0 mm (H) |
|---|---|
| Weight |
38.0 kg
|
| Application |
qPCR
|
|---|---|
| Brand |
Eonis™ Q
|
| Detection Modality |
Molecular
|
| Model |
Eonis™ Q96
|
| Regulatory Status |
CE-IVD marked
|
Video gallery






Resources
Are you looking for resources, click on the resource type to explore further.
The EONIS Q system simplifies and streamlines molecular testing for SMA and SCID with an innovative 3 step workflow.
Revvity provides everything you need to build a newborn screening workflow
This brochure provides a complete overview of newborn screening soluions provided by Revvity


How can we help you?
We are here to answer your questions.