One of the major challenges in treating psychiatric and neurodegenerative diseases is the delivery of targeted therapies across the blood-brain barrier (BBB). While our understanding of brain pathology has advanced significantly over the past two decades, therapeutic development has, unfortunately, lagged behind. This gap is largely due to the fact that most biologics and small molecules fail to penetrate the BBB at concentrations sufficient to exert meaningful therapeutic effects.
Take monoclonal antibodies, for example. These have shown great success in the treatment of cancer and autoimmune diseases due to their high specificity and customizable binding properties. However, their large size (~150 kDa) and physicochemical properties limit their ability to cross the BBB, restricting their application in central nervous system (CNS) disorders.
This limitation has spurred interest in alternative biologic formats, most notably, nanobodies.
The promise of nanobodies in psychiatric disorders
Nanobodies, derived from the heavy-chain-only antibodies of camelids, are small, single-domain antibody fragments that retain the high antigen specificity of conventional antibodies while being significantly smaller at just 15 kDa. Their small size, structural stability, and low immunogenicity make them highly attractive candidates for CNS drug development.
Crucially, some nanobodies have shown the ability to cross the BBB making them uniquely suited for brain-targeted therapeutics.
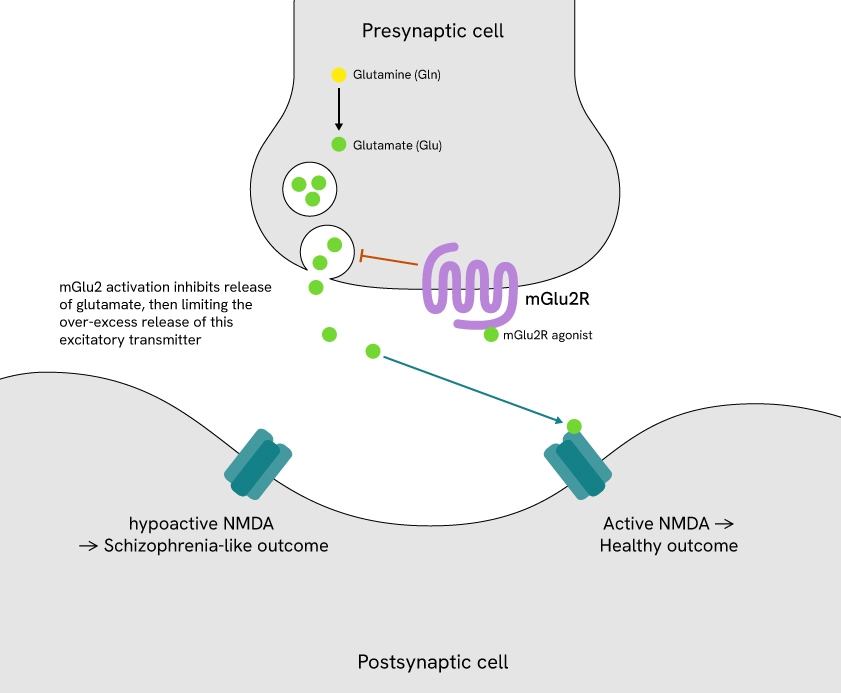
One particularly promising target is the metabotropic glutamate receptor 2 (mGlu2), a G protein-coupled receptor (GPCR) that regulates presynaptic glutamate release (Figure 1). Modulating mGlu2 activity can inhibit excessive glutamate release, a mechanism implicated in schizophrenia and related psychiatric disorders. This targeted approach helps restore glutamatergic balance without directly enhancing NMDA receptor activity, offering a more precise and controlled strategy to reduce glutamate overactivity.

Figure 1: mGlu2 in glutamatergic signaling.
Although several small-molecule mGlu2 agonists have advanced to clinical trials, many have failed due to issues such as poor receptor selectivity. These setbacks highlight the need for more selective, brain-accessible therapeutic strategies to modulate mGlu2 function.
Overcoming delivery barriers with a peripherally administered nanobody
In a recent Nature publication, Oosterlaken et al. reported the development of a bivalent biparatopic nanobody (DN13-DN1) that selectively binds mGlu2 at two distinct epitopes.1 Following peripheral injection, DN13-DN1 was able to cross the BBB, engage its target in the brain, and restore cognitive and behavioral deficits in two different mouse models of NMDA receptor hypofunction.
Impressively, a single dose of DN13-DN1 produced effects that lasted up to seven days, with no observable adverse effects or changes in mGlu2 receptor expression. As the authors note: “Such a long-term effect of the nanobody is a clear advantage over the use of small-molecule agonists of mGlu2, which showed effects only acutely and no effect when tested seven days after administration.”
This study provides compelling proof-of-concept that peripherally administered nanobodies can modulate CNS receptors with high specificity and durability, potentially overcoming the major delivery barriers that have limited past therapeutic strategies.
How Revvity technologies supported this research
Several advanced platforms from Revvity, including HTRF™ and Tag-lite™, were used to validate the function, distribution, and pharmacokinetics of DN13-DN1.
HTRF IP-One assay for measuring receptor activation
The researchers used Revvity's HTRF IP-One Gq Detection Kit to confirm that DN13-DN1 effectively enhanced mGlu2 receptor activity. This assay measures the intracellular accumulation of inositol monophosphate (IP1), a downstream marker of receptor activation through the Gq signaling pathway (Figure 2).
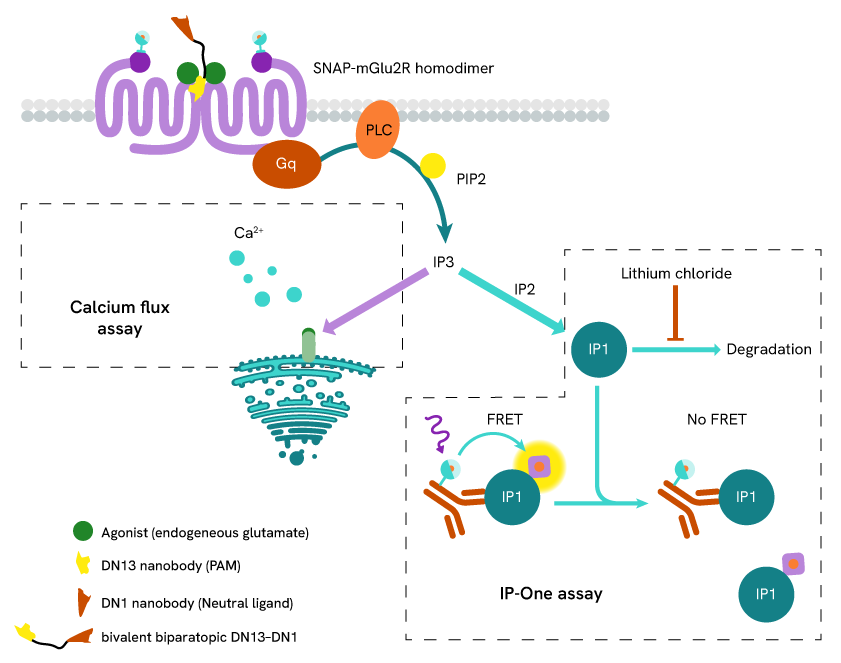
Figure 2: Assay principle of the HTRF IP-One Gq assay
The results demonstrated that DN13-DN1 functions as a positive allosteric modulator (PAM) of mGlu2. Notably, DN13-DN1 showed an almost 2-log improvement in PAM potency over the original DN13 nanobody, with the EC50 shifting from 101 nM to 1.76 nM.
Tag-lite technology for measuring binding and assessing pharmacokinetics
Tag-lite assays are based on labeling SNAP-tagged receptors and ligands with a FRET donor and acceptor, respectively, to allow for TR-FRET detection (Figure 3). In this study, the researchers used Tag-lite SNAP-Lumi4-Tb for two key readouts:
Binding affinity: To assess DN13-DN1 binding to SNAP-tagged mGlu2 receptors, the researchers labeled the receptors with Tag-lite SNAP-Lumi4-Tb fluorescent reagent. Increasing concentrations of 6xHis-tagged nanobodies and HTRF anti-6×His monoclonal antibody d2-conjugate were then added in the presence of agonist or antagonist. TR-FRET signals were measured, reflecting nanobody binding.
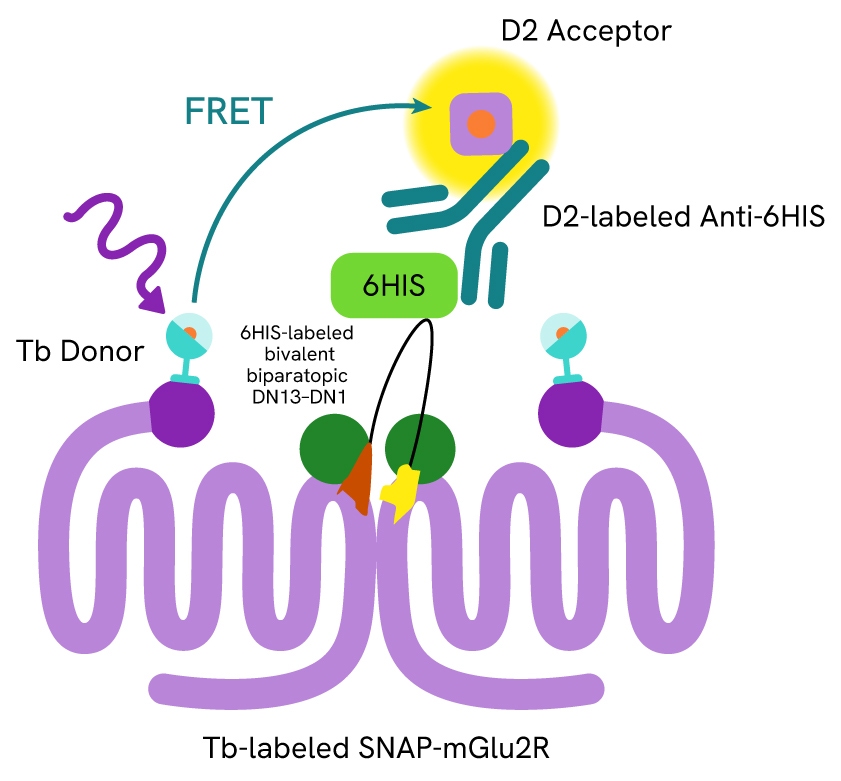
Figure 3: Format of the Tag-lite mGlu2 binding assay
This experiment showed that DN13-DN1 exhibited a 10-fold higher binding affinity for mGlu2 compared to DN13 alone, supporting the enhanced binding of the bivalent, biparatopic nanobody to the receptor.
Nanobody quantification in blood: To determine how long the DN13-DN1 and DN13 nanobodies remained in the bloodstream after injection, the authors used a modified version of the previously described TR-FRET binding assay. Plasma samples from 6xHIS-labeled nanobody-treated mice were collected at different timepoints after treatment (1h, 2h, 4h, 6h, 8h, 10h, 12h, and 24h) and added to cells expressing SNAP-mGlu2 receptors labeled with Tag-lite SNAP-Lumi4-Tb, in the presence of HTRF anti-6×His monoclonal antibody d2 conjugate. The resulting TR-FRET signals reflected nanobody-mGlu2R binding and were compared to a previously run linearity assay to estimate the remaining amount of nanobody in the blood for each sample.
They found that the larger DN13-DN1 nanobodies were cleared from the blood more slowly than the smaller DN13 nanobody, with DN13-DN1 still detectable up to 8 hours after the injection.
HTRF binding in brain tissue
To confirm that behavioral changes weren't due to changes in receptor levels, the researchers used Revvity's HTRF technology to detect and quantify mGlu2 receptors in brain tissue samples. Cells were incubated with two labeled nanobodies, DN10-d2 (acceptor) and DN1-Tb (donor). Each of the nanobodies targets a different site on mGlu2R, meaning a single receptor can bind both at the same time. This allows the two nanobodies to come into close proximity, which results in a FRET signal of intensity proportional to the amount of nanobody docking sites, i.e., receptors, available in the tissue sample.
They found no changes in receptor expression across various brain areas, suggesting that the behavioral effects observed were not due to changes in receptor numbers.
Spotlight on Revvity products
| Tool | Application | Impact |
|---|---|---|
| HTRF IP-One Gq Detection Kit | Measures IP1, a marker of mGlu2 receptor activation | Confirmed that DN13–DN1 potentiates mGlu2 signaling |
| Tag-lite SNAP-Lumi4-Tb | Labels SNAP-tagged receptors for TR-FRET | Enabled measurement of nanobody binding affinity and blood PK |
| HTRF Binding Assay with DN10-d2 and DN1-Tb | Quantifies endogenous mGlu2 receptors in tissue | Verified receptor levels were unchanged post-treatment |
Implications for next-generation CNS therapeutics
Beyond mGlu2, the implications of this study extend to the broader field of neuroscience drug development, with nanobody-based approaches showing great potential for circumventing the limitations of size and brain delivery that have historically hampered progress.
As the field advances, Revvity's Tag-lite and HTRF binding platforms will be essential for screening novel nanobodies, validating binding properties, and supporting functional studies, making them ideal for accelerating breakthroughs in neuroscience drug discovery.
Reference:
- Oosterlaken, M., Rogliardo, A., Lipina, T., Lafon, P. A., Tsitokana, M. E., Keck, M., Cahuzac, H., Prieu-Sérandon, P., Diem, S., Derieux, C., Camberlin, C., Lafont, C., Meyer, D., Chames, P., Vandermoere, F., Marin, P., Prézeau, L., Servent, D., Salahpour, A., Ramsey, A. J., … Rondard, P. (2025). Nanobody therapy rescues behavioural deficits of NMDA receptor hypofunction. Nature, 10.1038/s41586-025-09265-8. Advance online publication. https://doi.org/10.1038/s41586-025-09265-8
For research use only. Not for use in diagnostic procedures.


































