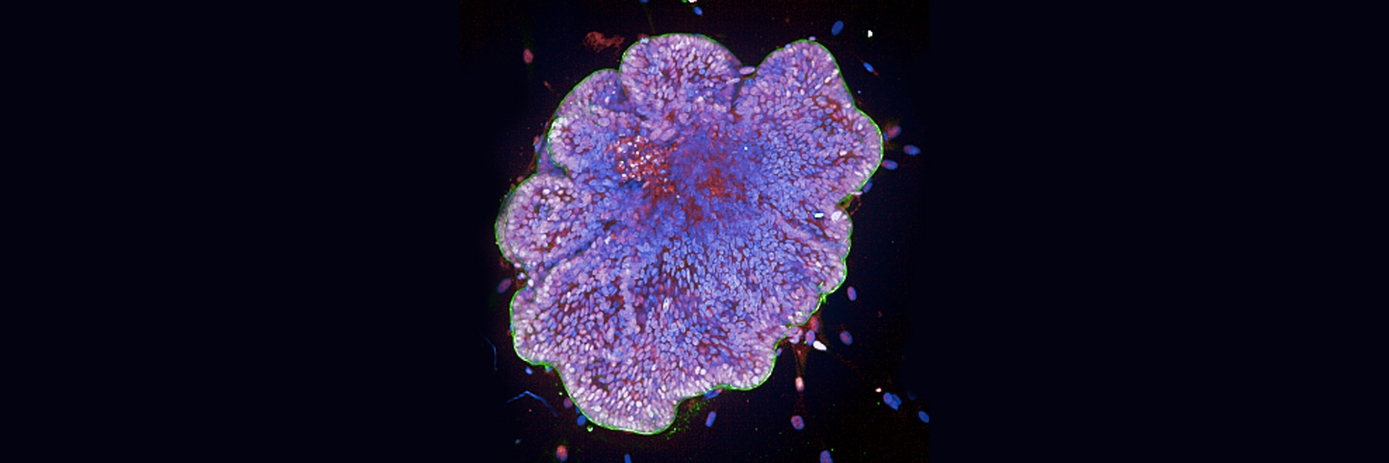
Organoids, miniaturized 3D cell models that recapitulate key aspects of human organs, are reshaping how researchers study biological processes, disease mechanisms, and treatment responses.
Derived from pluripotent or adult stem cells, these self-organizing tissue-like structures offer greater physiological relevance than traditional 2D cultures and provide human-specific insights that complement data from animal models.
The increasing demand for more predictive and human-relevant experimental systems has accelerated the development and adoption of organoids in diverse research areas, from basic biology to translational and clinical applications.
Where organoids are making an impact
With their ability to closely mimic tissue structure, function, and heterogeneity, organoids are offering opportunities to gain deeper insights across a wide range of research areas1,2:
- Precision medicine research: Patient-derived organoids enable ex vivo testing of therapeutic responses using an individual’s own cells, supporting the development of individualized treatment strategies and bringing the promise of personalized medicine closer to reality.
- Disease modeling: Organoids are enabling researchers to investigate complex disease phenotypes, including cancer, neurodegenerative disorders, and infectious diseases, under physiologically relevant conditions, facilitating the study of disease mechanisms and progression.
- Drug discovery and development: Organoids provide more physiologically relevant responses to compound perturbations, thereby enhancing screening accuracy and reducing the risk of costly late-stage failures in drug development.
- Toxicology and safety assessment: Organoids support more accurate safety profiling by replicating human tissue responses to chemicals and toxins, improving early detection of toxicity risks while reducing reliance on animal models.
- Regenerative medicine: Researchers are using organoids derived from an individual’s own stem cells to better understand organogenesis, with promising applications for transplantation therapies and tissue regeneration without immune rejection risks.
From complex models to clear insights
As organoids continue to transform research across multiple fields, extracting meaningful data from these models becomes crucial to their scientific value.
While their 3D structure and complexity make organoids more challenging to analyze than traditional cultures, researchers are successfully applying established laboratory techniques to study and interpret these advanced models.
Technologies like high-content imaging are being used to visualize organoid structure and morphology, while ATP-based luminescence assays provide quantitative assessment of viability and proliferation. Complementary techniques for cellular analysis include flow cytometry, which quantifies specific cell populations through surface marker detection after organoid dissociation, and single-cell sequencing, which reveals detailed gene expression profiles and heterogeneity at the transcriptomic level.
Integrating these techniques into research workflows supports the characterization of organoid systems and enhances the generation of meaningful data.
Whether you’re establishing organoid workflows or refining your methods, learning from real-world research can help maximize the impact of these sophisticated models.
Download our Organoid Analysis eBook to explore how researchers are successfully analyzing organoids and discover key techniques and tools used to study these complex 3D models.
For research use only. Not for use in diagnostic procedures.
Image courtesy of Romain Dautreppe and Nicolas Gatimel, Hôpitaux de Toulouse.
References:
- Yang S, Hu H, Kung H, Zou R, Dai Y, Hu Y, Wang T, Lv T, Yu J, Li F. Organoids: The current status and biomedical applications. MedComm (2020). 2023 May 17;4(3):e274. doi: 10.1002/mco2.274.
- Zhu Z, Cheng Y, Liu X, Ding W, Liu J, Ling Z, Wu L. Advances in the Development and Application of Human Organoids: Techniques, Applications, and Future Perspectives. Cell Transplant. 2025 Jan-Dec;34:9636897241303271. doi: 10.1177/09636897241303271.