Small RNAs are key regulators of gene expression, chromatin organization, stress responses, and genome integrity. Within this diverse landscape, PIWI‑interacting RNAs (piRNAs) have emerged as the most abundant class of small RNA in animal germline cells. They are present in vertebrates and invertebrates but absent in plants. According to piRBase (v3.0), millions of unique sequences are currently annotated as human piRNAs, highlighting a level of diversity and complexity that far exceeds that observed for microRNAs1. First described in Drosophila testes and mammalian germ cells, piRNAs are associated specifically to PIWI clade Argonaute proteins2,3.
Their defining function is genome defence. PIWI–piRNA complexes silence transposable elements, mobile genetic sequences whose uncontrolled activity can disrupt coding sequences, perturb regulatory regions, and induce DNA damage. Acting as a molecular immune system, piRNAs enforce silencing at multiple levels4,5. In this blog, we will provide an overview of current knowledge about this relatively underappreciated class of small RNAs.
Structure, biogenesis and expression
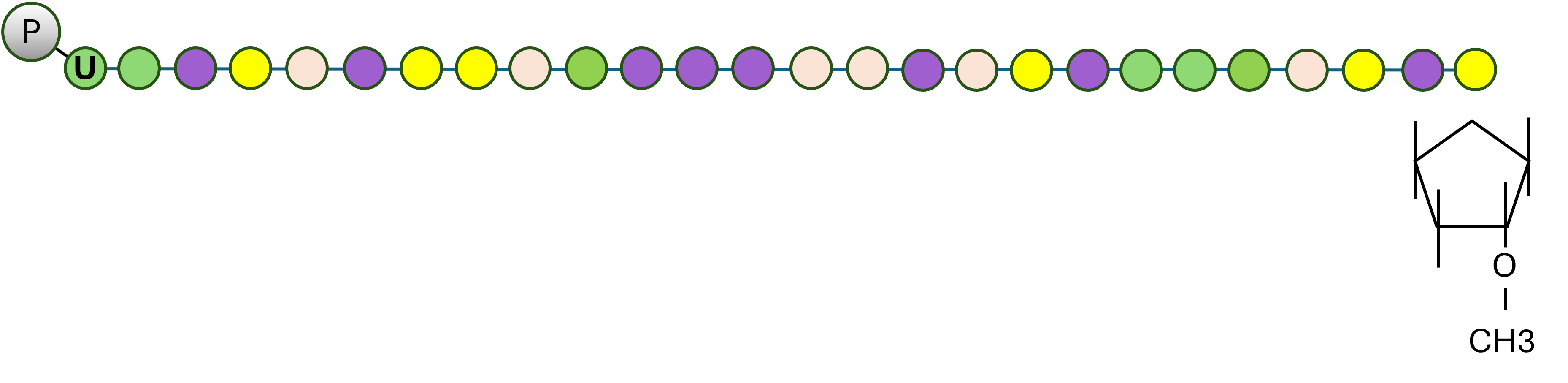
piRNAs are a distinct class of small RNAs defined by their length, nucleotide composition, and chemical modifications. They are typically 26–31 nucleotides long (as opposed to 21–24 nt in microRNA), have no clear secondary structure motifs and their biogenesis is independent of Dicer. A bias for a uridine at the 5’ end has been observed in piRNAs from both vertebrates and invertebrates3. A defining biochemical hallmark of piRNAs is the 2′-O-methyl modification at the 3′ end. This modification is introduced by the methyltransferase HENMT1 and protects piRNAs from exonucleolytic degradation, ensuring their stability in the cellular environment6. This stability allows piRNAs to persist longer than other small RNAs, making them particularly effective in sustaining long-term silencing of transposable elements.

Figure 1. Structural features of piRNAs
piRNA biogenesis begins with transcription of long single-stranded precursors from piRNA clusters, which are specialized genomic regions enriched in remnants of transposable elements. These precursors are cleaved by specific endonucleases, producing mature piRNAs that are loaded onto PIWI proteins7. Expression of piRNAs is highest in germline cells, where they play a central role in preserving genome integrity during spermatogenesis and oogenesis. In mammals, PIWI–piRNA complexes are not only required to cleave transposon transcripts (post-transcriptional silencing) but also direct de novo DNA methylation at transposable element loci, ensuring that silencing is stably inherited across generations (epigenetic silencing)8.
While germline enrichment is their most prominent feature, piRNAs are also found in somatic tissues at low levels, such as brain in mammals, where they may contribute to synaptic plasticity and memory formation, although findings remain debated9. Dysregulated expression of piRNAs and PIWI proteins has been reported in multiple cancer types, where they may influence proliferation, survival, and tumor progression. Because of their biochemical stability, tissue-specific expression patterns, and sequence diversity, piRNAs are now being investigated as promising biomarkers and potential therapeutic targets.10
Challenges in sequencing piRNAs
While small RNA sequencing has become the method of choice for profiling piRNAs, several technical and analytical challenges complicate their study. The 2′-O-methyl modification at the 3′ end reduces the efficiency of conventional adapter ligation, potentially biasing library composition. Size selection is also critical, as piRNAs overlap in length with other abundant small RNA fragments such as tRFs and Y-RNAs. Without precise enrichment, contaminating species can obscure piRNA detection. The NEXTFLEX Small RNA seq kit
 NEXTFLEX Small RNA Sequencing Kit V4
has been used to profile sperm small RNA species, including piRNA11.
NEXTFLEX Small RNA Sequencing Kit V4
has been used to profile sperm small RNA species, including piRNA11.
On the computational side, piRNAs often arise from large genomic clusters rich in repetitive elements, leading to a high proportion of multi-mapping reads that are difficult to assign unambiguously. Specialized computational pipelines exploit sequence features such as the 1U signatures, but the absence of a universally standardized reference database, unlike the well-curated miRBase for miRNAs, adds to the interpretive burden.
Conclusion
piRNAs exemplify the richness and complexity of the small RNA landscape. Their specialized biogenesis, germline enrichment, and ability to safeguard genome integrity set them apart from other small RNA classes, while their emerging roles in somatic biology and disease highlight their translational potential. From a technical standpoint, piRNAs challenge the limits of library preparation and bioinformatics, demanding careful optimization of workflows.
For laboratories already experienced with small RNA sequencing of miRNAs or tRFs, expanding into piRNA research represents an opportunity to explore one of the most dynamic areas of RNA biology.
References:
- Wang, J., et al (2022). piRBase: integrating piRNA annotation in all aspects. Nucleic Acids Res. 50(D1): D265-D272. doi: 10.1093/nar/gkab1012.
- Vagin, V.V., et al. (2006). A Distinct Small RNA Pathway Silences Selfish Genetic Elements in the Germline. Science 313, 320-324. doi:10.1126/science.1129333.
- Girard, A., et al. (2006). A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202. doi:10.1038/nature04917.
- Juliano C, et al (2011). Uniting germline and stem cells: the function of Piwi proteins and the piRNA pathway in diverse organisms. Annu Rev Genet. 45:447-69. doi: 10.1146/annurev-genet-110410-132541.
- Pritam, S., Signor, S. (2025). Evolution of piRNA-guided defense against transposable elements. Trends in Genetics, 41(5): 390-401. Doi:10.1016/j.tig.2024.11.011.
- Lim, S.L., et al. (2015) HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse. PLOS Genetics 11(10): e1005620. Doi:10.1371/journal.pgen.1005620.
- Watanabe T, et al (2011). MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev Cell. 20(3):364-75. doi: 10.1016/j.devcel.2011.01.005.
- Aravin AA, Bourc'his D. Small RNA guides for de novo DNA methylation in mammalian germ cells. Genes Dev. 2008 Apr 15;22(8):970-5. doi: 10.1101/gad.1669408.
- Rojas-Ríos, P., et al (2018). piRNAs and PIWI proteins: regulators of gene expression in development and stem cells. Development 1 145 (17): dev161786. doi: 10.1242/dev.161786
- Wu X, el al (2020). The Biogenesis and Functions of piRNAs in Human Diseases. Mol Ther Nucleic Acids. 21:108-120. doi: 10.1016/j.omtn.2020.05.023.
- Hamilton, M., et al. Assessing spermatozoal small ribonucleic acids and their relationship to blastocyst development in idiopathic infertile males. Sci Rep 12, 20010 (2022). https://doi.org/10.1038/s41598-022-24568-w
For research use only. Not for use in diagnostic procedures.


































