RNA interference using small interfering RNAs (siRNAs) has become a mainstay of functional gene characterization and has generated over a dozen FDA-approved therapeutics and drugs in late-stage clinical trials. Algorithms for selection of functional siRNA sequences were developed early on; however, some determinants for rational selection of siRNAs have remained elusive until just recently. A mechanistic study by Peter Wang in David Bartel’s lab sheds new light on slicing kinetics of the Argonaute enzyme central to RNA interference.
Soon after siRNAs were discovered in the early 2000’s they were quickly harnessed as a research tool which enabled the growing field of functional genomics. Rules had to be established for the selection of potent siRNAs to turn this discovery into useful technology: small RNA-directed knockdown of nearly any desired transcript. Early studies of siRNAs and microRNAs (which share many similarities to siRNAs), allowed for both rational design and machine learning-assisted selection of functional molecules.
Many features of potent siRNA duplexes were first published in 2004 by scientists at Dharmacon, Inc. (Reynolds, 2004). Some of these observations could be described by the known function of the active enzyme, Argonaute 2 (Ago2), the main effector of the RNA-Induced Silencing Complex (RISC) in mammalian cells. For instance, both low G/C content, as well as low internal stability at the “sense” strand 3′-terminus, aid Ago2 in unwinding the siRNA duplex and selecting the “correct” targeting strand for loading over the sense or passenger strand. However, some functional determinants were less easily explained, such as base preferences at certain positions in the duplex (positions 3, 10, and 13 in the sense strand, corresponding to positions 7, 10, and 17 in the targeting strand).
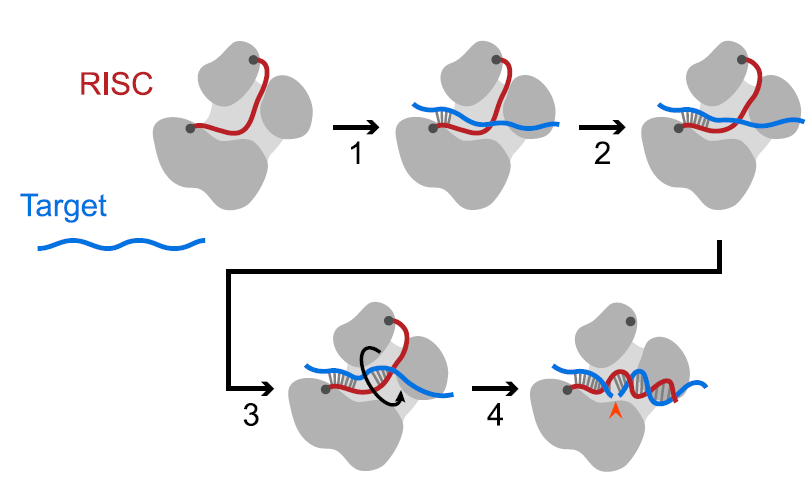
As a result of the work showcased in Wang, 2024, a more complete picture has emerged of the dynamic Ago2 complex. Slicing can now be more fully explained as a 4-step model (Figure 1 below) which involves binding in the seed region of the targeting strand, propagation through the seed, a second nucleation event to center the slicing target, and a helical rotation to complete base pairing throughout the site. Slicing kinetics are affected by those intermediate steps (including rearrangement inside Ago2) to form a complete duplex between the targeting strand: target mRNA and a favored conformation for slicing.
Figure 1 adapted from Wang, 2024 [published under CC-BY license]

The machine-learning approach of the original Dharmacon algorithm had predicted the cryptic preferences of an A at position 3, a U at position 10, and the absence of G at position 13, but could not yet rationally explain why these were beneficial for function. Thanks to this new and detailed biochemical study, we understood these design elements from a biophysical perspective, confirming that Dharmacon siRNA design remains state-of-the-art for potent gene knockdown.
Dive deeper into the science behind effective siRNA design of our Dharmacon reagents with our exclusive on-demand webinar including many relevant applications. Learn how these insights continue to advance functional genomics research and therapeutic development.
Read the full publication in Molecular Cell:
Wang PY, Bartel DP. The guide-RNA sequence dictates the slicing kinetics and conformational dynamics of the Argonaute silencing complex. Mol Cell. 2024 Aug 8;84(15):2918-2934.e11. doi: 10.1016/j.molcel.2024.06.026.
Read the original Dharmacon algorithm publication here:
Reynolds, A. et al. Rational design for RNA interference. Nature Biotechnol. 2004 Feb 1 doi:10.1038/nbt936.