Functional genomic screening and cell panel screening represent complementary methodologies that, when combined, create a powerful framework for novel target identification in therapeutic development. These orthogonal approaches generate rich, multi-dimensional datasets to strengthen target validation through independent confirmation.
What is functional genomic screening and why is it important?
Functional Genomic Screening (FGS) employs genetic perturbagens (e.g. CRISPRko, CRISPRi, CRISPRa, siRNA, ASOs) to allow researchers to scrutinize genetic interactions. When performed in combination with chemical perturbagens, these screens can probe drug-gene interactions to:
- Identify gene drivers for sensitivity or resistance phenotypes.
- Identify the involvement of cellular pathways.
- Provide understanding of drug mechanism of action (MOA).
The versatility of FGS lies in its flexible implementation formats. Researchers can choose between pooled and arrayed formats from small scale through to whole genome, with the choice driven by the screen parameters, such as assay type, cells utilized, scale, reagent type, and endpoint. Pooled screening is an excellent choice for larger, proliferation, or selection-based screens. Arrayed FGS provides the option of multiple phenotypic read-outs for an in-depth exploration using defined genetic (and chemical) perturbagens, including co-culture assay formats.
Why use cell panel screening?
Cell Panel Screening (CPS) provides a comprehensive, high-throughput platform for the evaluation of compound effects across diverse cellular contexts. This approach enables researchers to test chemical agents against multiple cell lines representing different tissues, genetic backgrounds, and disease states simultaneously.
By measuring phenotypic responses quantitatively, CPS can highlight patterns of sensitivity or resistance across cellular models. These patterns help researchers identify which specific cellular contexts respond favorably to treatment, guiding treatment strategy and understanding clinical therapeutic potential. Further versatility can be gained by combinational therapeutic screening (including with standard of care drugs) to explore synergistic relationships.
How to combine functional genomic and cell panel screening?
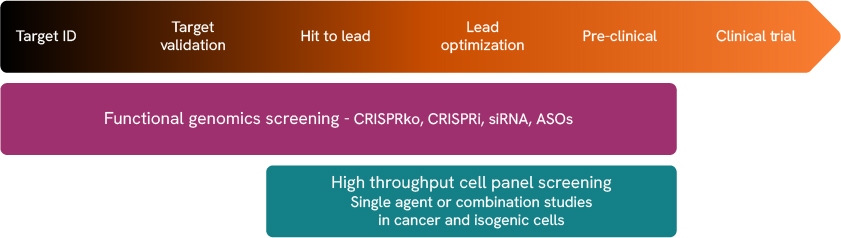
FGS and CPS are versatile tools that can be leveraged throughout the therapeutic development pipeline (Figure 1). To showcase how FGS and CPS can be utilized together to provide direct validation of screen hits, we performed a pooled CRISPR knockout (CRISPRko) screen using the PARP inhibitor, olaparib, followed by different CPS screening formats.1

Figure 1. Functional genomic screening and cell panel screening can support multiple phases of the therapeutic development pipeline.
We performed a negative selection pooled CRISPRko screen to identify genes that when disrupted, enhance sensitivity to PARP inhibition. This approach reveals synthetic lethal interactions by detecting the depletion of specific knockout cells within the treated population. Our analysis identified several key sensitivity determinants, including ATM, members of the FANC pathway, and components of the RNaseH2 complex. These findings aligned well with previously published CRISPRko PARP inhibitor screens examining PARP inhibitor sensitivity in different cellular contexts.2
To profile cell line and tissue-dependent responses, we conducted CPS across 326 cancer cell lines treated with a PARP inhibitor. This viability-based assay enabled classification of cell lines as either responders or non-responders. Comparative analysis of these groups using DepMap data revealed genetic features associated with PARP sensitivity. Notably, the pathways identified through this approach showed significant overlap with hits from our initial CRISPR screen, providing orthogonal validation.
For further confirmation, we performed a focused combination screen using 10 disease-relevant cell lines. PARP inhibitor treatment was performed in combination with inhibitors targeting various pathways identified from the pooled CRISPRko screen. Here, synergy scores were profiled between drug combinations, validating hits, and identifying cell line vulnerabilities and potential treatment strategies.
Conclusion
Our work demonstrates the significant advantages of integrating Functional Genomic Screening (FGS) and Cell Panel Screening (CPS) methodologies for accelerated target validation in therapeutic development. This combined approach creates a robust framework for identifying and confirming potential drug targets.
FGS excels at providing mechanistic insights by comprehensively identifying genes and pathways that influence drug response. CPS then serves as an efficient validation platform, rapidly confirming these findings across diverse cellular contexts while simultaneously building a profile of potential clinical applications. This bidirectional workflow enhances confidence in identified targets through orthogonal validation.
Importantly, this integrated strategy can be implemented with equal effectiveness in reverse sequence. Researchers may begin with CPS to identify responsive cellular phenotypes, followed by FGS to elucidate the underlying molecular mechanisms driving these responses. This flexibility allows teams to leverage existing data or infrastructure while still benefiting from the complementary nature of both approaches.
Learn more about how our preclinical services team can help advance your research with our functional genomics and cell panel screening services.
References
- Blanck M, et al. “A Flexible, Pooled CRISPR Library for Drug Development Screens.” The CRISPR journal vol. 3,3 (2020): 211-222. doi:10.1089/crispr.2019.0066.
- Zimmermann M, et al. “CRISPR screens identify genomic ribonucleotides as a source of PARP-trapping lesions.” Nature vol. 559,7713 (2018): 285-289. doi:10.1038/s41586-018-0291-z
For research use only. Not for use in diagnostic procedures.