Guide RNAs, or single-guide RNAs (sgRNAs), are indispensable tools in functional genomics and transcriptome modulation. Their programmable nature and their ability to direct Cas9 or other Cas-endonucleases to specific genomic loci underpins the utility of these systems in knockout studies, transcriptional activation/repression, RNA editing, and more (Wu et al, 2014; Kim et al, 2024). This blog reviews the main scenarios where sequencing of sgRNAs by NGS is relevant. It also provides suggestions on the appropriate strategies to prepare libraries to sequence sgRNAs, based on their structural features.
Stably expressed viral delivery systems
Verification of sgRNA sequences is critical whenever guides are delivered from lentiviral or adeno-associated viral (AAV) vectors, since these platforms support long-term co-expression of the nuclease and guide (Liu et al, 2017). Sequencing ensures that the intended guide is present, intact, and functional. This is important for experimental reproducibility. Below, we outline a few use cases where sgRNA sequencing provides important quality control and risk mitigation.
Plasmid-library quality control prior to packaging
Sequencing the plasmid pool before virus production confirms library complexity, uniform guide representation, and the absence of synthesis or cloning artifacts, providing a reference baseline for subsequent screen analysis (Zhou et al, 2023).
Pooled lentiviral screens
Genome-wide or focused CRISPR screens rely on high-complexity lentiviral libraries that integrate one sgRNA per cell. Post-selection sequencing of the integrated guide region is essential for deconvolving barcode counts, calculating enrichment/depletion statistics, and assigning gene-level phenotypes (Mathiowetz et al, 2023; Bock et al, 2022).
Single or low multiplex delivery
Even when only one or a few guides are packaged, sequencing of the expressed sgRNA is recommended to confirm functionality and rule out point mutations or recombination events introduced during cloning or reverse transcription, which could lead to confusion about functional read-outs or off-target assessments.
Ex vivo cell delivery
Ex vivo therapeutic approaches where patient-derived cells are modified (e.g., CAR-T, genome-edited T cells or modified stem-cells), require sequence verification of gRNAs prior to re-infusion. This ensures accurate on-target editing and avoids unintended batch variability. It is also a requisite for submissions and regulatory oversight from US Food Drug Administration, European Medicines Agency and the Pharmaceuticals and Medical Devices Agency (FDA, 2002; EMA 2018; PMDA 2019).
In vivo delivery
For in vivo gene editing, especially using AAV or non-integrating viral vectors, regulatory expectations are higher due to direct patient exposure and prolonged expression risks. Complete sequencing of the gRNA cassette ensures guide integrity, validates identity, and rules out deleterious insertions or recombination events (FDA, 2022; EMA 2018; PMDA 2019)
Manufacturing Control Applications
Control of guide identity
Synthetic sgRNA production demands rigorous identity verification. Sequencing confirms the nucleotide composition and the correct targeting region, eliminating ambiguity introduced by chemical synthesis errors, mislabelling, or oligonucleotide cross-contamination.
Control of guide purity
Each nucleotide addition during solid-phase RNA synthesis has a coupling efficiency below 100%. As the RNA length increases, the likelihood of producing full-length molecules declines, and truncated byproducts (e.g., N–1, N–2 species) accumulate. For RNAs of approximately 100 nucleotides, such as sgRNAs, the reduction in yield is already significant (Rees et al., 2021). Even with purification methods like HPLC or PAGE, it remains technically challenging to separate full-length sgRNAs from closely sized truncated products, especially for sequences prone to strong secondary structure or base-specific retention behaviors (Kanavarioti, 2019). The emergence of sequencing as a quality control step offers an orthogonal approach to assess molecular purity and detect undesired species.
Strategies for sgRNA sequencing
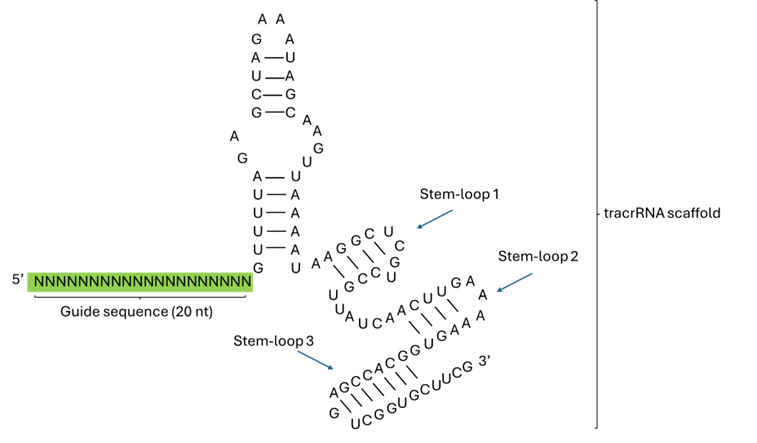
The length and structure of sgRNAs vary depending on the CRISPR-Cas system: Cas9 sgRNAs are typically ~100 nt, while Cas12 and Cas13 crRNAs are shorter, around 40–60 nt. In Cas9 systems, which are commonly used, the sgRNA consists of a 17–20 nucleotide spacer sequence that directs target recognition, and an ~80–100 nt tracrRNA-derived scaffold that enables Cas9 binding and activation (Jinek et al, 2012) (Figure 1). In contrast, Cas12a uses a ~42–44 nt sgRNA that combines a 20–24 nt spacer with an ~18–22 nt direct repeat-derived scaffold forming a hairpin (Zetsche et al, 2015). Cas13 systems, which target RNA rather than DNA, use sgRNAs composed of a ~24–30 nt spacer and a ~24 nt scaffold, and the Cas13 protein self-processes these guides to form an active ribonucleoprotein complex (Abudayyeh et al., 2017).

Figure 1. Structure of a Cas9 sgRNA. Modified from Shumega et al (2024).
Due to their modest size, sgRNAs are ideally suited for the library preparation methods develop for small RNA-seq.
 NEXTFLEX Small RNA Sequencing Kit V4
Traditionally used for sequencing of miRNA and other small RNA molecules, they can be effectively adapted for sgRNA.
NEXTFLEX Small RNA Sequencing Kit V4
Traditionally used for sequencing of miRNA and other small RNA molecules, they can be effectively adapted for sgRNA.
One adaptation consists in adjusting the final size selection step, which by default selects for inserts <100 nucleotides in length. Alternative size selection protocols are readily available to capture longer molecules, encompassing sgRNAs.
A second adaptation concerns the chemical nature of the 5' end of the sgRNA. Canonical small RNA naturally contains a 5' monophosphate and this feature is required for library preparation. If the sgRNA that we want to study does not contain a 5'-monophosphate, then an enzymatic step upstream of library prep is required, for which guidelines are readily available and can be provided upon request. Chemically synthesized sgRNAs contain usually a 5'-monophosphate or 5'-hydroxyl group, depending on the final deprotection steps used. On the other hand, sgRNA transcribed in vitro or expressed in the cells from Pol III promoter will contain a 5'-triphosphate group (Xie et al, 2017).
Concluding Remarks and Future Perspectives
The importance of accurate and comprehensive sequencing of guide RNAs cannot be overstated. Robust quality control ensures the reliability and reproducibility of experimental outcomes. As genome editing applications continue to grow in complexity and precision, advancements in sequencing technologies and optimized library preparation strategies will be key to supporting both innovation and regulatory compliance.
References:
- Wu X, Kriz AJ, Sharp PA. (2014). Target specificity of the CRISPR-Cas9 system. Quant Biol. (2):59-70. doi: 10.1007/s40484-014-0030-x.
- Kim HS, Kweon J, Kim Y. (2024). Recent advances in CRISPR-based functional genomics for the study of disease-associated genetic variants. Exp Mol Med. 56(4):861-869. doi: 10.1038/s12276-024-01212-3.
- Liu C, Zhang L, Liu H, Cheng K. (2017). Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J Control Release. 266:17-26. doi: 10.1016/j.jconrel.2017.09.012. Zhou, Y., Wang, L., Lu, Z. et al. (2023). Optimized minimal genome-wide human sgRNA library. Sci Rep 13, 11569. doi:10.1038/s41598-023-38810-6.
- Mathiowetz AJ, Roberts MA, et al. (2023). Protocol for performing pooled CRISPR-Cas9 loss-of-function screens. STAR Protoc. 4(2):102201. doi: 10.1016/j.xpro.2023.102201.
- Bock, C., Datlinger, P., Chardon, F. et al. (2022). High-content CRISPR screening. Nat Rev Methods Primers 2, 8. doi:10.1038/s43586-021-00093-4.
- U.S. Food and Drug Administration. (2022). Human gene therapy products incorporating human genome editing: Guidance for industry. https://www.fda.gov/media/156894/download.
- European Medicines Agency. (2018). Guideline on quality, non-clinical and clinical aspects of gene therapy medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-clinical-aspects-gene-therapy-medicinal-products_en.pdf.
- Pharmaceuticals and Medical Devices Agency. (2019). Guidelines on ensuring the quality and safety of gene therapy products. https://www.pmda.go.jp/files/000235837.pdf.
- Rees HA, Minella AC, et al (2021). CRISPR-derived genome editing therapies: Progress from bench to bedside. Mol Ther. 29(11):3125-3139. doi: 10.1016/j.ymthe.2021.09.027.
- Kanavarioti, A. (2019). HPLC methods for purity evaluation of man-made single-stranded RNAs. Sci Rep 9, 1019 doi:10.1038/s41598-018-37642-z.
- Jinek, M. et al., A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 337,816-821(2012). DOI:10.1126/science.1225829
- Zetsche, B., et al. (2015). Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell, 163(3), 759–771. https://doi.org/10.1016/j.cell.2015.09.038
- Abudayyeh, O., Gootenberg, J., et al. (2017) RNA targeting with CRISPR–Cas13. Nature 550, 280–284 . doi:10.1038/nature24049.
- Shumega AR, Pavlov YI, et al. (2024) CRISPR/Cas9 as a Mutagenic Factor. Int J Mol Sci. 2(2):823. doi: 10.3390/ijms25020823.
- Xie, C., Chen, YL., Wang, DF. et al. (2017) SgRNA Expression of CRIPSR-Cas9 System Based on MiRNA Polycistrons as a Versatile Tool to Manipulate Multiple and Tissue-Specific Genome Editing. Sci Rep 7, 5795 doi: 10.1038/s41598-017-06216-w.
For research use only. Not for use in diagnostic procedures.