Drug development is an expensive and high-risk endeavor, with many promising candidates failing late in development. While multiple factors contribute to these setbacks, one of the most significant, but often overlooked, is unexpected immune system interactions.
A compound may demonstrate strong therapeutic potential in preclinical models, only to trigger unpredicted immune activation or suppression when tested in humans. By the time these issues arise, significant resources have been invested and years of research may be lost.
Advanced immune profiling technologies can help mitigate these risks by providing a deeper understanding of immune interactions early in the development process. These tools enable researchers to predict immune responses more accurately, improving candidate selection and increasing the likelihood of clinical success.
The importance of primary human cells in immunogenicity testing
A major challenge in preclinical testing is the reliance on cell lines. While these models offer valuable insights, they do not fully capture the complexity of the human immune system. As a result, they may fail to accurately predict how a compound will behave in the human body, potentially leading to incomplete assessments of its safety and efficacy.
Primary human immune cells, isolated directly from human donors, offer greater physiological relevance and reflect the genetic diversity of real-world patient populations. Screening therapeutic candidates with these cells provides deeper insights into how compounds interact with the immune system, helping scientists to evaluate potential secondary effects and safety risks before clinical trials begin.
Overcoming immune profiling challenges
Traditional immune assays, while valuable, can be time consuming, require large reagent volumes, and often lack the depth needed to fully understand the impact of a compound on the immune system. These inefficiencies can limit throughput, increase costs, and make it difficult to scale up immune profiling. This potentially leaves critical stones unturned in the drug development process.
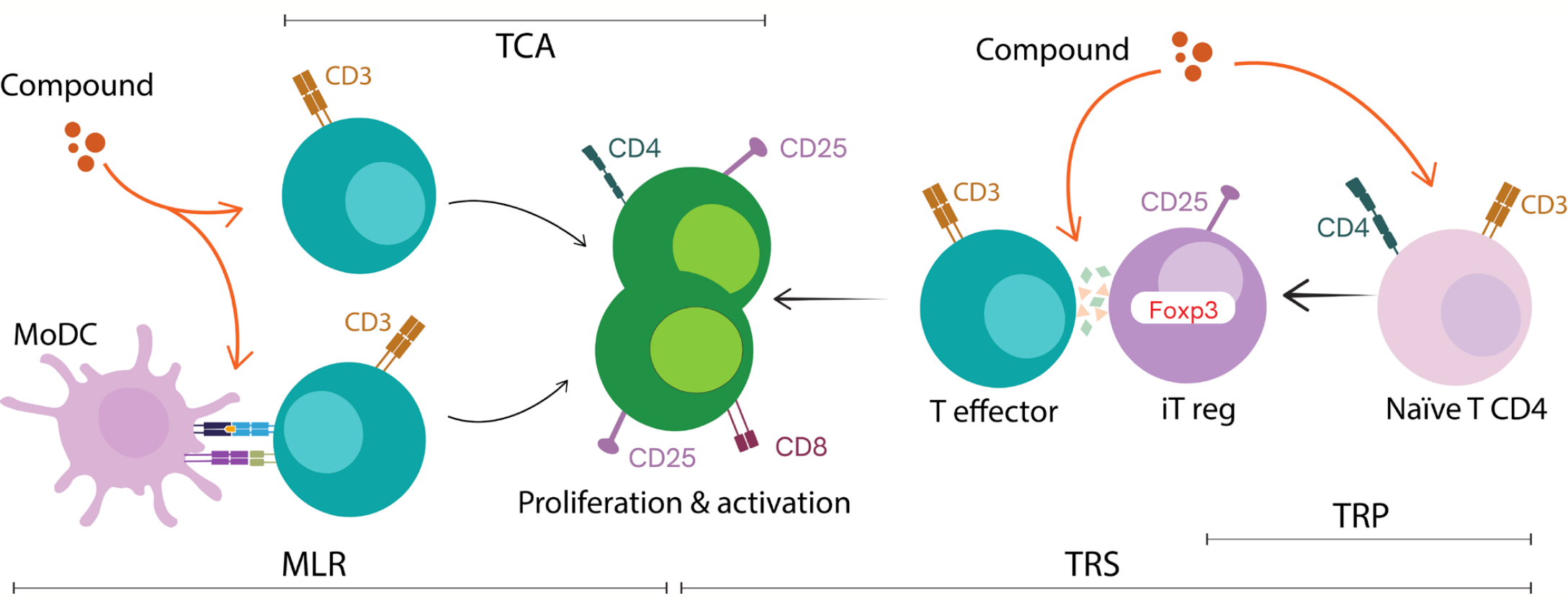
Recent innovations in immune profiling technology now allow researchers to assess multiple aspects of immune function in parallel. Technologies such as LEGENDplex™ multiplex immunoassays facilitate the measurement of multiple biomarkers in a single sample, providing a broader view of immune responses. Additionally, ImmuSignature™ assays (Figure 1) simulate in vitro immune environments, enabling researchers to analyze how compounds interact with immune cells under physiologically relevant conditions.
These tools enhance immune profiling by improving efficiency, reducing reagent consumption, and generating deeper insights into immune system interactions. By incorporating these assays into immune profiling workflows, researchers can gain a more detailed understanding of how drug candidates interact with the immune system, improving confidence in candidate selection and minimizing unexpected immune reactions in later-stage trials.

Figure 1: Diagram of ImmuSignature assays. It comprises four complementary models of therapeutic analysis over T cell physiology, centered on quantifying the proliferation and activation of T CD4+ and TCD8+ cells. The T cell activation assay (TCA) is a simple dose-response layout of compounds over isolated T cells, while the mixed lymphocyte reaction (MLR) analyses the compound effect over a co-culture of an antigen-presenting cell (MoDc) and T lymphocytes. On the other hand, compounds can also be investigated in their effect on the polarization of naïve T cells to a regulatory phenotype, an iTreg polarization (TRP) assay. Furthermore, compounds can also be challenged in their influence of the iTregs to modulate the regulatory function over T effector cells in a suppression assay (TRS).
Conclusion
Incorporating advanced immune profiling tools into the early stages of drug development helps mitigate the risks associated with immune system interactions, offering a more physiologically relevant view of how therapeutic candidates might perform in human trials.
By reducing costs, improving throughput, and enhancing the depth of immune analysis, technologies like LEGENDplex™ and ImmuSignature™ allow researchers to identify high-quality leads with greater precision, accelerating the journey from discovery to clinical success. To explore immune profiling solutions for your research, download our latest application note on LEGENDplex™ multiplex assays and ImmuSignature™ immune profiling technologies.
For research use only. Not for use in diagnostic procedures.


































