The discovery of novel immunomodulatory compounds and effective vaccine adjuvants is a critical focus in immunology research. However, traditional methods for identifying such compounds often face limitations in throughput, scalability, and reproducibility. High-throughput screening (HTS) has transformed this field, serving as an essential tool for the rapid evaluation of thousands of compounds to assess cytotoxicity, immunological, and phenotypic effects. To address this challenge, Chew et al [1] has developed a HTS protocol utilizing human peripheral blood mononuclear cells (PBMCs) to model immune responses.
This innovative approach combines the sensitivity of AlphaLISA™ immunoassays with flow cytometry to measure key cytokine secretion from stimulated PBMCs, including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-10 (IL-10), along with cell surface activation markers in response to compound exposure. The use of cryopreserved PBMCs in this study ensures reproducibility, while the integrated workflow offers a rapid and scalable approach to discovering new immunomodulators.
Four-step high-throughput workflow:
- Step 1: Thawing cryopreserved PBMCs on Day 0
- Step 2: Splitting the supernatant and cells on Day 3 to prepare for cytokine measurement using AlphaLISA and flow cytometry
- Step 3: Cells are then fixated and stained on a microplate for analysis
- Step 4: Quantitative readouts for cell surface marker expression via flow cytometry along with measurement using AlphaLISA technology to evaluate cytokine secretion levels (e.g., TNF-α, IFN-γ, IL-10) from the collected supernatants to determine the immunomodulatory potential of the compounds assessed.
What is AlphaLISA technology?
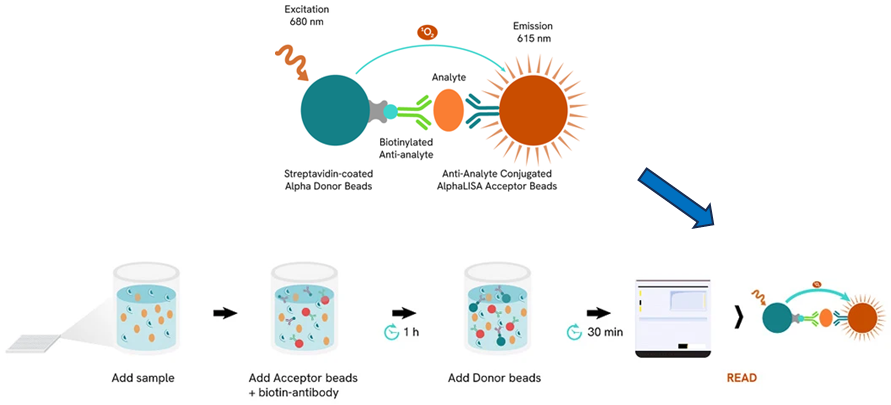
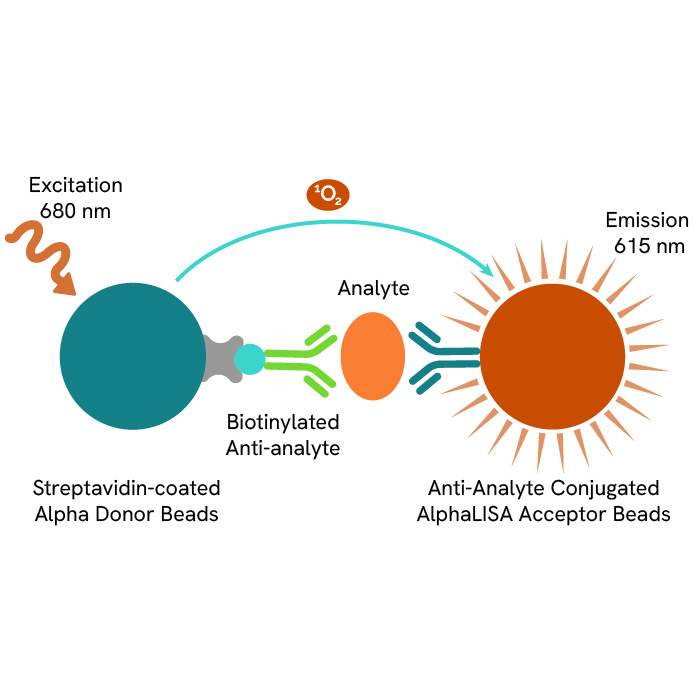
AlphaLISA (Amplified Luminescent Proximity Homogeneous Assay) is a fast, highly sensitive, homogeneous, no-wash assay platform performed in a microplate format that has been integrated into this screening protocol to measure immune responses accurately. The platform utilizes Alpha Donor and Acceptor beads, which, when brought into proximity by the binding of the target analyte, produce a luminescent signal upon excitation (Figure 1).

Figure 1. Schematic of AlphaLISA technology, a fast, highly sensitive, homogeneous, no-wash assay platform utilizing Donor and Acceptor beads for precise detection of molecules of interest. The workflow includes sample and bead addition, luminescence detection via a plate reader, and signal readout.
AlphaLISA’s cutting-edge technology offers several advantages:
- High sensitivity and specificity: AlphaLISA provides a robust signal with minimal background interference, allowing for the precise quantification of low-abundance cytokines.
- Homogeneous, no-wash assay format: The no-wash protocol simplifies the workflow, reducing assay time and minimizing potential errors associated with washing steps.
- Compatibility with HTS: The technology is amenable to automation and miniaturization, making it suitable for high-throughput applications.
The study demonstrated that cryopreserved PBMCs stimulated in autologous plasma for 72 hours using small molecule libraries elicited distinct cytokine profiles for TNF-α, IFN-γ, and IL-10, which were precisely measured using AlphaLISA technology. The results obtained highlight the platform’s precision and utility in high-throughput detection of immune responses. Positive controls (R848 and ODN-2395) demonstrated significant cytokine induction compared to negative controls (DMSO), validating the assay’s sensitivity and reproducibility. Overall, these results support the use of AlphaLISA for high-through immune response detection and profiling experimental compounds. Key findings:
-
AlphaLISA enables precise and sensitive cytokine profiling:
The study demonstrated that cryopreserved PBMCs stimulated in autologous plasma for 72 hours using small molecule libraries elicited distinct cytokine profiles for TNF-α, IFN-γ, and IL-10, which were precisely measured using AlphaLISA technology. The results obtained highlight the platform’s precision and utility in high-throughput detection of immune responses. Positive controls (R848 and ODN-2395) demonstrated significant cytokine induction compared to negative controls (DMSO), validating the assay’s sensitivity and
-
Comprehensive immune activation insights via flow cytometry:
Complementary flow cytometry data enriched the cytokine findings by providing phenotypic insights into immune activation. Cell surface markers, including CD80, CD86, HLA-DR, and OX40, were analyzed to characterize activation states across various immune cell subsets. These markers indicated activation of specific immune cell subsets, including monocytes, B cells, and T cells. Compounds inducing heightened expression of these markers were flagged as potential immunomodulators. The dual-platform approach of integrating AlphaLISA and flow cytometry solidified the reliability and depth of the immunological profiling.
Conclusion:
This study showcases the power of AlphaLISA technology in a high-throughput setting for immunomodulator screening. By combining cytokine detection with flow cytometry, the authors developed a multiplexed platform capable of identifying potential therapeutic candidates efficiently. AlphaLISA’s advantages, including its no-wash format and scalability, underscore its potential for broad applications in immunological research and beyond.
Future directions for this study may include expanding the cytokine panel and integrating other cell types to explore a wider array of immune responses. Overall, this study reaffirms AlphaLISA’s role as a transformative tool in biomedical research.
AlphaLISA Kits Used in Chew et al.’s Study*:
- TNF-a AlphaLISA Detection Kit, 5,000 Assay Points; Product Number: AL208: AlphaLISA High Performance Human TNFα Detection Kit, AL3157
 AlphaLISA Human TNF-α High Performance Detection Kit, 100 Assay Points
AlphaLISA Human TNF-α High Performance Detection Kit, 100 Assay Points
- IFN-g AlphaLISA Detection Kit, 5,000 Assay Points; Product Number: AL217: AlphaLISA High Performance Human IFNγ Detection Kit, AL3153
 AlphaLISA Human IFN-γ High Performance Detection Kit, 100 Assay Points
AlphaLISA Human IFN-γ High Performance Detection Kit, 100 Assay Points
- IL-10 AlphaLISA Detection Kit, 5,000 Assay Points; Product Number: AL218: AlphaLISA Human IL-10 High Performance Detection Kit, AL3159
 AlphaLISA Human IL-10 High Performance Detection Kit, 100 Assay Points
AlphaLISA Human IL-10 High Performance Detection Kit, 100 Assay Points
*Note: All kits that were used in the study have been replaced with AlphaLISA High Performance kits.
Read the full protocol here.
Learn more about Revvity's AlphaLISA technology.
References:
- Chew K, Lee B, Ozonoff A, Smith JA, Levy O, Dowling DJ, Van Haren S. A protocol for high-throughput screening for immunomodulatory compounds using human primary cells. STAR Protoc. 2023 Sep 15;4(3):102405. doi: 10.1016/j.xpro.2023.102405. Epub 2023 Jul 13. PMID: 37453068; PMCID: PMC10365952.