CSF1R-ALSP: A devastating neurological disease
CSF1R-related adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (CSF1R-ALSP) is a rare microgliopathy characterized by progressive white matter degeneration and neurological decline, with poor prognosis [1,2]. CSF1R-ALSP is primarily driven by loss-of-function mutations in the colony-stimulating factor 1 receptor (CSF1R) gene, leading to impaired microglial survival, proliferation, and homeostasis. Despite its severe clinical course consisting of cognitive decline, motor dysfunction, and psychiatric symptoms, CSF1R-ALSP lacks approved therapies, with misdiagnosis common due to overlapping symptoms with Alzheimer’s disease or multiple sclerosis [3].
CSF1R-mediated signaling pathways
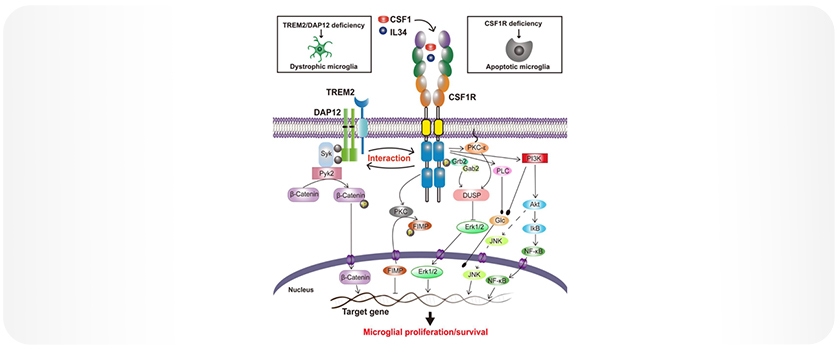
CSF1R is a receptor tyrosine kinase activated by the ligands CSF1 and IL-34. Upon ligand binding, CSF1R dimerizes and undergoes autophosphorylation at several intracellular tyrosine residues, including Tyr561, Tyr699, Tyr708, and Tyr723. This phosphorylation initiates downstream signaling cascades, such as PI3K/Akt, ERK1/2, and JNK, which regulate microglial proliferation, survival, and differentiation (Figure 1).

Figure 1: Proposed CSF1R-mediated signaling in microglial survival and proliferation. Upon stimulation by its ligands CSF1 or IL-34, CSF1R undergoes rapid dimerization and autophosphorylation at key tyrosine residues, leading to the activation of PI3K/Akt, JNK, and ERK1/2 pathways that regulate microglial survival and proliferation. CSF1R also interacts with the TREM2/DAP12 complex, further supporting microglial survival. Note: Figure adapted from Hu et al., 2021, licensed under CC BY 4.0 [4].
Disruption of CSF1R signaling, as observed in loss-of-function mutations, leads to microglial depletion and subsequent neurodegeneration. This highlights the critical role of CSF1R in maintaining microglial homeostasis and underscores its therapeutic relevance not only in rare diseases like CSF1R-ALSP but also in more common neurodegenerative disorders. Consequently, CSF1R has emerged as a promising target for drug development aimed at modulating microglial function and mitigating neurodegeneration.
Exploring CSF1R as a therapeutic target
Recent clinical trials, such as the Phase 2 IGNITE trial involving iluzanebart—a human monoclonal IgG1 antibody agonist of human TREM2 (hTREM2) designed to selectively activate TREM2 [2]—have highlighted both the potential and challenges in this approach. Despite encouraging preclinical results [1], the trial did not meet its efficacy endpoints.
A June 2025 update from Vigil Neuroscience reported that iluzanebart was safe and well-tolerated at both 20 and 40 mg/kg doses with expected pharmacokinetics. However, it failed to improve key biomarkers or clinical metrics, leading to the discontinuation of the long-term extension study. Following Sanofi’s acquisition of Vigil, development of iluzanebart was halted in favor of alternative TREM2-targeting approaches, including the small-molecule agonist VG-3927. These results emphasize the ongoing need for robust, scalable tools capable of elucidating microglial signaling with precision, particularly in pathways as complex and dynamic as CSF1R.
AlphaLISA™ SureFire® Ultra™ provides a validated and effective approach for measuring intracellular signaling events with high sensitivity and throughput. This no-wash, homogeneous immunoassay platform delivers robust performance in complex cellular models, making it an ideal tool for advancing CSF1R-targeted drug discovery and assessing pathway modulation in disease-relevant systems.
Results: Measuring CSF1R activation with AlphaLISA SureFire® Ultra
In a study using THP-1 cells stimulated with macrophage colony-stimulating factor (M-CSF, 100 ng/mL), a key ligand that activates CSF1R and supports macrophage survival and function, robust phosphorylation of CSF1R was observed at multiple tyrosine residues following treatment (Figure 2). Phosphorylation peaked at 5 minutes post-treatment, with fold changes of 18.6-fold at Tyr561, 14-fold at Tyr699, 3-fold at Tyr708, and 11.7-fold at Tyr723. Concurrently, total CSF1R levels decreased over time, consistent with receptor internalization or degradation following activation.
Phosphorylation of downstream effectors ERK1/2 at Thr202 and Tyr204 (5-fold) and AKT1/2/3 at Ser473 (6-fold) at 5 minutes further confirmed activation of key signaling pathways involved in microglial and macrophage function.
Overall, the assays exhibited high sensitivity, reproducibility, and dynamic range, highlighting the AlphaLISA SureFire® Ultra platform as a reliable and precise tool for measuring CSF1R signaling and evaluating pathway modulation in complex cellular models.
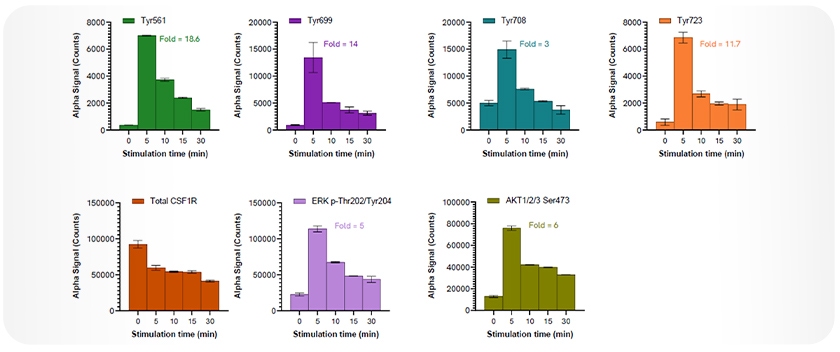
Figure 2: Total and phosphorylated levels of CSF1R following M-CSF stimulation. THP-1 cells were stimulated with M-CSF (100 ng/mL) for the indicated time points. Phosphorylation of CSF1R at Tyr561 (18.6-fold), Tyr699 (14-fold), Tyr708 (3-fold), and Tyr723 (11.7-fold) peaked at 5 minutes post-treatment. Total CSF1R levels decreased over time. Activation of downstream signaling was confirmed by phosphorylation of ERK1/2 (Thr202/Tyr204; 5-fold) and AKT1/2/3 (Ser473; 6-fold), also peaking at 5 minutes. All measurements were performed using AlphaLISA SureFire® Ultra assays, with a single lysate used to measure multiple targets. Equivalent to ~20,000 cells per data point.
Conclusion
Revvity provides essential tools to advance the understanding and exploration of CSF1R signaling. The high sensitivity of AlphaLISA SureFire® Ultra assays enables precise analysis of key phosphorylation events, allowing researchers to monitor signal transduction, evaluate drug efficacy, and accelerate the development of targeted therapies.
By supporting detailed investigation of CSF1R pathways, Revvity’s technologies play a critical role in advancing therapeutic strategies for CSF1R-related disorders. As research in this field progresses, these innovative solutions remain central to driving discovery and improving outcomes for patients with CSF1R-ALSP and related neurodegenerative diseases.
About AlphaLISA technology:
AlphaLISA (Amplified Luminescent Proximity Homogenous Assay) is a bead-based, no-wash homogeneous assay platform, enabling the detection of analytes and a dynamic range of biological interactions, including protein-protein, protein-peptide, and protein-small molecule interactions. This technology utilizes streptavidin-coated Alpha Donor beads and AlphaLISA Acceptor beads. Upon excitation, a singlet oxygen molecule is generated in the Donor bead that triggers a series of chemical reactions in the Acceptor bead, resulting in the emission of light at 615 nm, which is directly proportional to the amount of analyte or binding events within the sample. Alpha technology allows for the direct detection of molecules of interest in a homogeneous format, eliminating the need for time-consuming wash steps.
AlphaLISA SureFire® Ultra (ALSU) is a subset of AlphaLISA technology offered by Revvity, which uses streptavidin-coated Donor beads and Acceptor beads coated with a proprietary CaptSure™ agent. ALSU enables direct measurement of phosphorylated or total intracellular proteins directly from cell lysates. With no wash steps, a streamlined workflow, and compatibility with high-throughput platforms, ALSU assays are ideal for cell signaling studies, including kinase activity, pathway profiling, and drug screening. These assays are widely used in neuroinflammation, oncology, and immunology research to provide robust and reproducible data, even from limited or sensitive cell populations (Figure 3). Note: For research use only. Not for use in diagnostic procedures.
Explore AlphaLISA SureFire® Ultra kits for CSF1R and related targets:
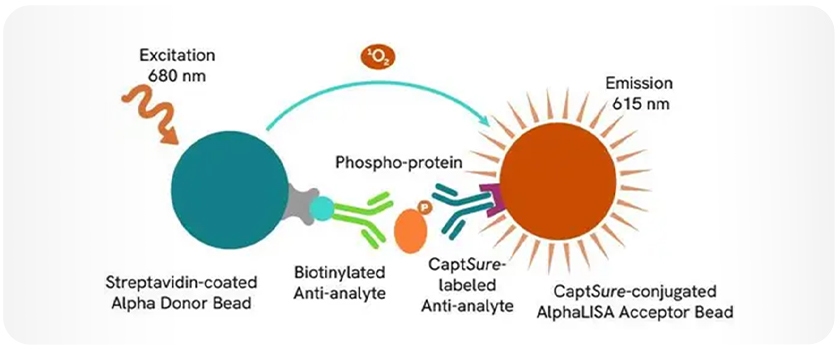
Figure 3. AlphaLISA SureFire Ultra assay schematic.
- Phospho-CSF1R (Tyr699)
- Phospho-CSF1R (Tyr708)
- Phospho-CSF1R (Tyr723)
- Phospho-ERK1/2 (Thr202/Tyr204)
- Phospho-AKT1/2/3 (Ser473)
Additional resources:
- AlphaLISA Technology:
- Revvity's Solutions for Neuroinflammation
- Revvity’s TREM2 Application Note: AlphaLISA SureFire® Ultra: elucidating TREM2/DAP 12 signaling in neuroinflammation
References:
- Larson, K.C., Gergits, F.W., Renoux, A.J. et al. Rescue of in vitro models of CSF1R-related adult-onset leukodystrophy by iluzanebart: mechanisms and therapeutic implications of TREM2 agonism. J Neuroinflammation 22, 26 (2025). https://doi.org/10.1186/s12974-025-03346-1.
- Meier A, Papapetropoulos S, Marsh A, Neelon K, Stiles D, O'Mara R, Thackaberry EA, Colonna M, Rajagovindan R. Phase 1, First-In-Human, Single-/Multiple-Ascending Dose Study of Iluzanebart in Healthy Volunteers. Ann Clin Transl Neurol. 2025 May;12(5):1065-1076. https://doi.org/10.1002/acn3.70033. Epub 2025 Apr 1. PMID: 40166927; PMCID: PMC12093347.
- Konno T, Kasanuki K, Ikeuchi T, Dickson DW, Wszolek ZK. CSF1R-related leukoencephalopathy: A major player in primary microgliopathies. Neurology. 2018 Dec 11;91(24):1092-1104. https://doi.org/10.1212/WNL.0000000000006642. Epub 2018 Nov 14. PMID: 30429277; PMCID: PMC6329328.
- Hu B, Duan S, Wang Z, Li X, Zhou Y, Zhang X, Zhang YW, Xu H, Zheng H. Insights Into the Role of CSF1R in the Central Nervous System and Neurological Disorders. Front Aging Neurosci. 2021 Nov 15;13:789834. https://doi.org/10.3389/fnagi.2021.789834. PMID: 34867307; PMCID: PMC8634759. Attribution Note: Figure 1 is adapted from Hu et al., 2021, and is licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).